Team members: Alikhani, J., Forero, L. G., Goetsch, H., Moore, J., Ward-Gokhale, L.
Mentors: Joe Eisenberg, Jade Mitchell
Problem Statement
Introduction
Honey bees are critical pollinators for a variety of crops -- 87 of the highest global production volume crops depend on animal pollination and honey bee species like Apis mellifera are considered the most cost effective and practical option for commercial pollination, according to Klein, et al.[1]. Overall, honey bee pollination contributes approximately $15 billion to the United States economy, according to Calderone et al.[2]. Exposures to which honey bees are particularly sensitive can be devastating to biodiversity and industrial production of bee-derived and pollinated products, thus involving a wide variety of stakeholders from honey consumers to multinational agrochemical producers. This risk assessment focuses on the combined risk to honey bee populations from neonicotinoid pesticide exposure and Nosema ceranae infection.
In order to understand the relationships between neonic exposure, Nosema infection, and honey bee individual-level and colony-level health, this risk assessment exercise incorporates a dose response model with uncertainty, a transmission model of fungal parasite Nosema spp. from bee to bee, and the impact of neonicotinoid dose on transmission and mortality rate. We considered the effects of one bee hive and extrapolate transmission to other hives.
Hazard Identification
In the late 20th century, neonicotinoid pesticides were developed to combat the organophosphate and organochlorine resistance developing in pest species. This class was also an important innovation due to its lack of overt, acute toxic effect in mammals, according to Jeschke and Nauen[3]. Since neonicotinoids, also known as neonics, were brought to market in the 1990s, the class has expanded to comprise around one-third of the global insecticide market. The most common method of application is to coat seeds in neonics before selling and planting them, and so the plants will take up the chemical throughout their vasculature when developing and pollen and nectar will both contain some neonic residues. Soil and water contamination, including dust generated before seeds are planted, may present additional sites of exposure, according to Godfray et al.[4].
Since these compounds have become so dominant in modern agriculture, scientists and apiculturists have noted that bee populations are diminishing and may be more frequently infected with parasitic organisms than had been the case earlier in the 20th century. Data from laboratory studies indicate that exposure to neonicotinoid pesticides can adversely affect a honey bee’s immune system, even at sublethal neonicotinoid concentrations, according to Brandt et al.[5]. The microsporidian fungal parasite Nosema ceranae is a common pathogen of the honey bee, though its direct health effects are not well-understood. Nosema infection has been correlated with bee dysentery, reduced hive productivity, and low-level mortality of individual or small groups of bees, according to Malonea and Gatehouse[6].
It has recently been shown that, when combined, these two stressors lead to enhanced bee mortality and immune system impairment (Alaux et al.[7]) and greater frequencies of colony collapse, according to Vidau et al.[8]. Stakeholders concerned with the combined impact of these two agents include the agrochemical and commodity crop industries, specialty crop producers reliant on honey bee pollination, beekeepers and honey producers, and consumers of bee-pollinated or bee-derived products.
Exposure Assessment
In order to model dose-response relationships and the transmission of Nosema between bees, literature values were employed as reasonable exposure level to neonicotinoid pesticides (5 ppb imidacloprid, according to Pettis[9]) and reasonable intake level of Nosema spores (2.0 * 10^5 spores/bee, according to Alaux[7].
Pollen contamination has been observed at levels ranging from 6.2-206.0 ppb imidacloprid in environmental sampling, and this risk assessment is designed to address concerns about low-dose, sublethal neonicotinoid levels so modeling with a level near but slightly lower than environmental concentrations is expected to be reasonable, according to Codling[10]. Further research could model the effects of more neonicotinoids or a wider range of doses.
The spore dose employed in this risk assessment was an adequate dose to achieve infection in all bees by five days post-administration. Further research could model the effects of different spore dose levels, including low doses at which not all bees may become infected.
\begin{align*} \frac{dS_j}{dt} = - \beta S_j I_j + \mu(S_j + I_j + R_j) - \mu S_j - \rho S_i + \frac{\rho}{n-1} \sum_{i=1 j \neq 1}^{n} S_i \end{align*}
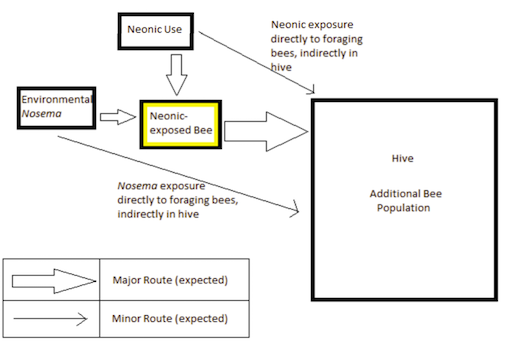
Figure 1: The exposure pathways by which the first bee and then its hivemates are exposed to neonicotinoids and Nosema spores.
Dose Response
We identified a previous study that conducted a dose-response experiment with honeybees and Nosema, according to Fries[11]. In that experiment, increases in Nosema spore inoculation led to increases in percent of bees infected. Upon extracting the data from this experiment, we fit the data to exponential and Beta-Poisson equations. These equations were optimized to minimize the total deviance by changing parameters of each question, k for exponential and alpha and N50 for Beta-Poisson. Excel could not solve the exponential function, so we selected the Beta-Poisson model to represent our data.
We attempted to incorporate uncertainty within our Beta-Poisson dose response model in two ways -- jackknifing and bootstrapping. For the Bayesian jackknifing, one of the original data points from the Fries[11] dose response data was removed and the alpha and N50 parameters were re-calculated. This was done for all five of the original data points. Alpha and N50 were assumed to be lognormally distributed, and their lognormal distributions were assumed to have means represented by the alpha and N50 of the entire Fries[11] data set. The standard deviations of the lognormal distributions were assumed to be equal to the jackknifed data. Attempts to generate the 95% confidence interval for the dose response model from a randomly generated, lognormally distributed data set with the given alpha and N50 means and standard deviations were unsuccessful, seemingly due to computational familiars.
Risk Characterization
Bootstrapping was employed for uncertainty analysis of the dose-response relationship. Both exponential and beta-poisson curves were fitted to the experimental data, and uncertainty bands were obtained by applying the bootstrapping method to the experimental data. The Exponential and Beta-Poisson model curves produced Minimum Deviance values of 61.1 and 16.5 respectively. Chi-squared critical test also showed that beta Poisson model is the best fitting model.
That gives the %95 confidence interval as:
\begin{align*} ln(\alpha) = [-1.05 \ -0.13] ln(N_{50}) = [3.87 \ 5.02] \end{align*}
In the transmission models, with increasing neonic concentration the bees die faster, however, we do not see a major effect of neonic concentration on the total number of infected and dead bees.
Figures 2 through 6 represent the results from considering the effect of neonic concentrations on the transformation and mortality due to infection. Figure 2 shows the number of susceptible bees declines over time - a higher concentration of neonic corresponds to a greater population decline compared to no neonic exposure over the same time frame. Figure 3 and 4 show the percent of infected bees and percent of infected and dead bees over time, respectively. As expected, Fig. 5 shows that the number of dead bees over time increases with an increase in neonic concentration. In Figure 6, to extend the transmission model for more than one hive, a second hive was included, assuming that hive contained no infected bees at the simulation start time; the exchange of bees between hives is incorporated by considering four different values of (between 0 and 1% probability that a bee moves from hive 1 to hive 2 and vice versa). Results in figure 5 show that increasing in the value of leads to a higher rate of infection over time in hive 2.
\begin{align*}\frac{dI_j}{dt} = \beta S_j I_j - \gamma I_j - \mu_i I_j - \mu I_j - \rho I_i + \frac{\rho}{n-1} \sum_{i=1 j \neq 1}^{n} I_j \end{align*}
Figure 2 - Role of different neonicotinoid concentrations in modulating rate of mortality due to infection () and the direct transfer rate () (Output is S, number susceptible bees).
\begin{align*} \frac{dR_j}{dt} = \gamma I_j - \mu R_j - \rho R_i + \frac{\rho}{n-1} \sum_{i=1 j \neq 1}^{n} R_i \end{align*}
Figure 3 - Role of different neonicotinoid concentrations in modulating rate of mortality due to infection () and the direct transfer rate () (Output is I, percent of infectious bees).
\begin{align*} \beta = bee -to -{bee\ contact\ rate\ in\ the\ hive} \times \frac{d(Risk)}{d(Dose)} at\ Dose = 0 \end{align*}
Figure 4 - Role of different neonicotinoid concentrations in modulating rate of mortality due to infection () and the direct transfer rate () (Output is percent of infectious plus dead bees)
\begin{align*} \frac{dP}{dD}=\alpha \frac{2^{(1/\alpha) -1}}{N_{50}} \end{align*}
Figure 5 - Number of dead bees exposed to different concentrations of neonicotinoids when both rate of mortality due to infection () and direct transfer rate () are affected by the presence of the insecticide. (Output is number of dead bees).
Figure 6 - Role of different neonicotinoid concentrations in modulating likelihood of leaving hive () rate (Output is the number of infected bees in Hive 1 and Hive 2 when changes).
The likelihood of colony collapse with application of current levels of neonicotinoid pesticides can be compared with the estimated worth of honey bee pollination to the United States economy ($15 billion as of 2009, according to Calderone et al.[2]) when monetizing the level of appropriate intervention or remediation strategies.
Risk Management & Communication
Based on the results of the risk assessment, we will recommend specific limits for neonicotinoid pesticide application which are estimated to minimize the risk of bee hive collapse. Other recommendations may include modification of bee hives’ proximity to commodity crops treated with neonicotinoid pesticides.
Risk Perception
Approximately one-third of the food we consume is pollinated somewhere in the production chain, so this issue is of interest to food producers, beekeepers, chemical producers, and also the general public. Even though Nosema infection does not pose a direct risk to human health, bee populations affected by Nosema and through greater susceptibility to Nosema by neonicotinoids will affect the sustainability of food production. Therefore resources and effort are warranted to better understand and potentially develop mitigation strategies around the effect of combined neonicotinoid and Nosema exposure to honey bees.
Risk Communication Strategy
In communicating with the public while keeping all relevant audiences in mind, risk managers and communicators addressing this issue should always first state that the relationship between neonic use and Nosema infection requires further study but may be harming honeybee populations. Consumers should be reassured that no adverse health outcomes for humans are caused by exposure to Nosema ceranae in the environment. There is some evidence of developmental and neurological effects from chronic exposure to concentrations of neonicotinoids found on/in crops that neonics have been demonstrated to present a decreased occupational hazard relative to legacy compounds like organophosphate or organochlorine pesticides, according to Klein, et al.[1] and Cimino et al.[12]. Consumer groups will also likely prefer messaging that includes a potential solution or mitigation strategy, and so the risk manager should also discuss how support for farms that do not use neonics may help reverse the trend.
Risk communication becomes a bit more fraught in the context of the food and chemical industries, because encouraging the public to think one way or another about their products may influence profits and public perception of those industries. These stakeholders should be encouraged to support research into colony collapse disorder and population maintenance strategies for honey bees, including the potential benefit of cooperating between commodity crop and pollinated crop producers to increase the spatial separation between neonic-treated crops and long- or short-term pollinator colonies.
References
- (2007). Importance of pollinators in changing landscapes for world crops.
- (2012). Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992-2009.
- (2008). Neonicotinoids - from zero to hero in insecticide chemistry..
- (2014). A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators.
- (2015). The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.).
- (1998). Effects of Nosema apis infection on honey bee (Apis mellifera).
- (2010). Interactions between Nosema microspores and a neonicotinoid weaken honey bees (Apis mellifera).
- (2011). Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honey bees previously infected by Nosema ceranae.
- (2013). Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae.
- (2016). Concentrations of neonicotinoid insecticides in honey, pollen, and honey bees (Apis mellifera L.) in central Saskatchewan, Canada.
- (1988). Infectivity and multiplication of Nosema apis Z. in the ventriculus of the honey bee.
- (2017). Effects of neonicotinoid pesticide exposure on human health: a systematic review.
 QMRA
QMRA