General overview
Acanthamoeba spp. are free-living amoeba (FLA) that have been commonly found in freshwater, tap water, and recreational water. Acanthamoeba spp. are capable of causing a variety of infections, including Acanthamoeba keratitis, an eye infection that has been associated with contect lens usage and cornea damage, and Granulomatous Amebic Encephalitis (GAE), a serious central nervous system infection that primarily affects immunocompromised individuals (Marciano-Cabral & Cabral, 2003; Visvesvara, Moura, & Schuster, 2007)[2] [1]. Considering these infections, some exposure routes of concern for Acanthamoeba spp. are the corneal and intranasal exposure routes.
Dean et al. (2020)[2][1] fit dose response models to data from previously conducted animal studies for Acanthamoeba spp. and the corneal and instranasal exposure routes. These models are an important step towards characterizing the risk associated with FLA like Acanthamoeba for drinking water-relevant exposure scenarios. More detailed descriptions of the datasets, fitting methods, model evaluation, and results can be found in the published article: http://dx.doi.org/10.1111/risa.13603
Recommended Model
For the intranasal exposure route, the exact beta-Poisson model fit to the pooled data from Experiments 3 and 4 is the recommended model. The successful pooling of the model gives additional confidence for applying the dose response relationship to additional Acanthamoeba strains and species.
[2] Marciano-Cabral, F., & Cabral, G. (2003). Acanthamoeba spp. as agents of disease in humans. Clinical Microbiology Reviews. https://doi.org/10.1128/CMR.16.2.273-307.2003
References
| ID | # of Doses | Agent Strain | Dose Units | Host type | Μodel | Optimized parameters | Response type | Reference |
|---|---|---|---|---|---|---|---|---|
| Acanth_Intranasal_Pooled | 9 | A. castellanii HN-3 and A culbertsoni A1 | no of trophozoites | mice | beta-Poisson |
a = 0.245 N50 = 19357 (beta=1215) |
death |
N50 = 19357 (beta=1215)
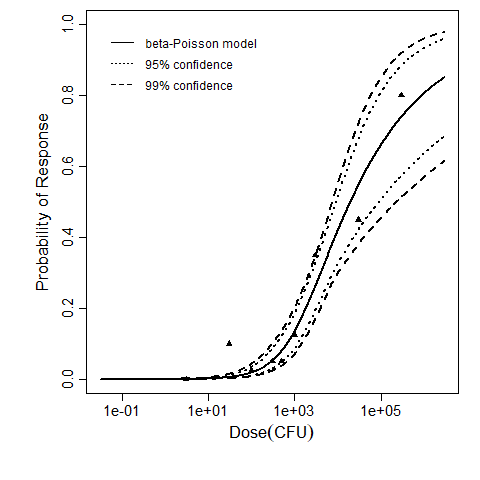
The same exposure route and endpoint was evaluated for Experiments 3 and 4 (Cerva, 1967b; Culbertson et al. 1966). A pooling analysis was attempted and successful. The beta-Poisson model provided a good fit to the pooled data and is shown below in Figure 1. Note: both the exact and approximate beta-Poisson models were fit to the data. The figures shown below and the csv file of bootstrapped parameter replicates are for the best fitting parameters of the exact beta-Poisson model. The successful pooling of multiple datasets generally increases the confidence in the estimated model parameters.
Figure 1: Plot of the beta-Poisson model fit to the pooled Experiments 3 and 4 with upper and lower 95% and 99% confidence
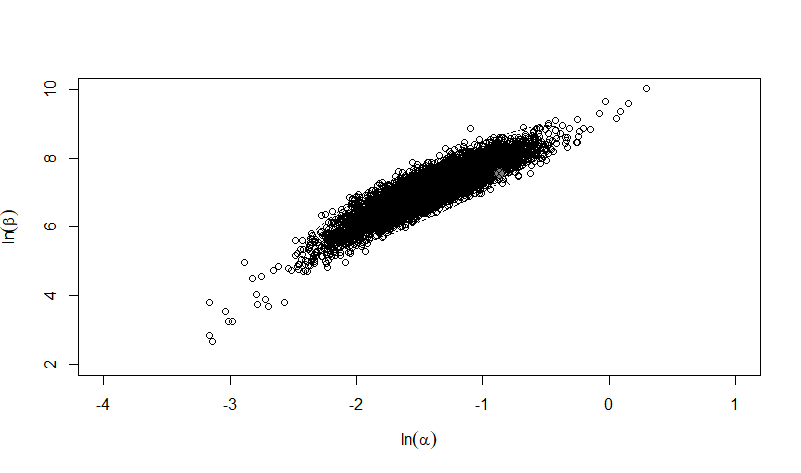
Figure 2: Uncertainty plot of the 10,000 paired bootstrap replicates of alpha and beta for the pooled beta-Poisson model.
Cerva, L. (1967b). Intranasal, Intrapulmonary, and Intracardial Inoculation of Experimental Animals with Hartmanella castellanii. Folia Parasitologica (Praha), 14, 207–215.
Culbertson, C. G., Ensminger, P. W., & Overton, W. M. (1966). Hartmannella (Acanthamoeba): Experimental Chronic, Granulomatous Brain Infections Produced by New Isolates of Low Virulence. The American Journal of Clinical Pathology, 46(3), 305–314.
 QMRA
QMRA