Problem Statement
The United States military has a goal of “net zero installations” known as the Net Zero Initiative. The initiative is a holistic strategy to use best practices for managing energy, water and waste at Army installations (US Department of Defense). Ultimately, the goal is for a military installation to be self-sustaining, with little to no impact on the resources in the area of installation.
Currently, potable water is brought in for use on military bases for activities such as laundry, showering, and cleaning. One approach toward the Net Zero Initiative goal is to reuse wastewater within the installation, rather than bringing in potable water to the installation for use. In other words, water used for purposes such as showering, laundry, or washing, could be collected, treated, and stored for future use.
Based on data about shower water delivery systems and typical characteristics of greywater, we assume that Legionella and Giardia will be present in the untreated greywater at the military installation, according to Blanky et al. Birks et al. Birks and Hills Friedler et al. and Leoni et al.. Though the water would be treated before use, military personnel have the potential to be exposed to pathogens in the reused water during showering via inhalation (Legionella) and incidental ingestion (Giardia). Data reported in the literature indicates that the amount of Legionella and Giardia in greywater is highly variable (Table 2). The aim of this risk assessment is to determine the health effects, if any, associated with using treated Class II water for showering on U.S. military bases, and introduce management strategies to reduce illness when necessary.
Problem Formulation
Water is a limited resource in military operations, and the US military is striving to meet a policy of Net Zero reused water. The US Army uses treated wastewater in various capacities, and is interested in incorporating the use of greywater when showering. The use of treated wastewater will help reduce the burden of water resource availability and meet the self-sufficient requirements of US military bases. Using class II water for showering and other purposes has possible health concerns that would defeat the benefits of conserving water, including a possible increase in the probability of infection.
Having more than 0.4% of the military personnel ill with a respiratory condition or more than 0.5% ill with a gastrointestinal illness is above the suggested level of weekly disease and non-battle injuries recommended by the Joint Chiefs of Staff (Rose). In this project, the aim is to assess the microbial risks associated with reusing water for showering on a military installation. We will consider the role of incidental ingestion of water, inhalation of water aerosols, and the role of biofilm in the water distribution system in our assessment. Risk of infection by Giardia or Legionella is possible through other activities as well, such as maintaining the water treatment system, or person-to-person transmission, respectively, but this analysis will focus on only the risks directly related to showering.
Hazard Identification
There are several opportunistic pathogens that could be present in used shower water, such as Aeromonas, Pseudomonas, Staphylococcus, Mycobacteria, protozoa, and viruses. Since the population at the military installation is assumed to be relatively healthy (e.g., not immunocompromised or elderly), we selected pathogens that have greater potential to affect a healthy population. The risks of ingestion of Giardia and inhalation of Legionella spp. were evaluated because these are the primary routes of exposure associated with these pathogens.
Giardia
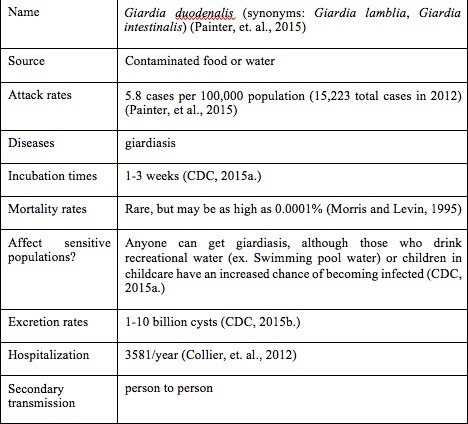
Giardia is a waterborne parasite that causes giardiasis and is spread through the feces of the host. Giardiasis is the most commonly diagnosed parasitic disease in the United States, with most cases being diagnosed in the summer months (between June to October) (Painter, et al.). The Giardia parasite has two forms: cysts (a hard shell-like covering containing the Giardia parasite) and trophozoites (Figure 1). Trophozoites are not as hardy as the cystic form and will not survive long in the environment. However, the cysts are more resistant, and can survive up to several months outside the host (Centers for Disease Control Prevention (CDC)). Though both forms are found in excreted feces of infected individuals, humans become infected only by swallowing Giardia cysts. The primary infection route for Giardia is through drinking improperly treated water that contains the cyst, including drinking water, recreational water, or other water sources such as ponds or rivers. Ingestion of contaminated foods can also lead to giardiasis. As few as ten cysts need to be swallowed in order to cause illness. Once infected, a person may shed between 1-10 billion cysts daily through their feces (CDC). Symptoms of giardiasis include gastrointestinal distress, such as stomach and abdominal cramping, gas, and diarrhea. These symptoms can lead to dehydration and weight loss, particularly in women and children (CDC). Other symptoms of giardiasis include itchy skin, hives, and swelling of the eye and joints, though these symptoms are less common. In most cases, those infected with Giardia will begin to show symptoms within one to three weeks of becoming infected, though some show no symptoms (CDC). Giardiasis typically lasts between two to six weeks in individuals who were healthy before becoming infected. Prescribed medications, such as metronidazole or tinidazole, can help decrease the time that symptoms last (CDC). Aside from avoiding contaminated foods and water, other ways to prevent giardiasis include practicing good hygiene and avoiding contact with feces. Table 1 shows the basics of the Giardia parasite.
Table 1. Giardia basics
Legionella spp
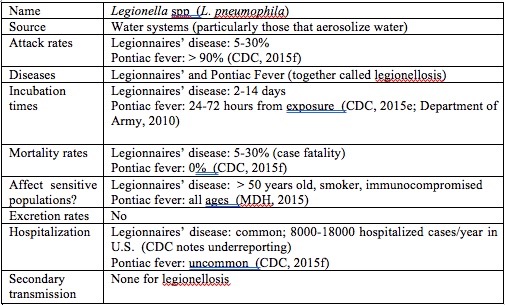
Legionella are gram negative bacterium that grow best in water at temperatures between 32°C and 45° C. When aerosolized, such as during a shower or spray irrigation, Legionella can be inhaled and become deposited in the lung. If an infection is established, diseases such as Legionnaires’ disease or Pontiac Fever, collectively called legionellosis, can result. Legionnaires’ disease is characterized by high fever, muscle aches, headache, fatigue, loss of appetite and coughing (Minnesota Department of Health (MDH); MDH). Those most at risk for Legionnaires’ disease include individuals who are over age 50, smokers, males, and people who have chronic lung disease or are immunocompromised (CDC). Legionnaires’ disease has a 2-14 day incubation period and an attack rate of 5%. The case fatality rate is 5-30% (CDC). Legionnaires’ disease is not transmitted from person to person.
Pontiac Fever is a milder, flu-like illness, also caused by exposure to Legionella. While the symptoms are similar to those of Legionnaires’ disease, there are no particular risk factors for Pontiac Fever. In other words, everyone is at risk (MDH), including the relatively healthy personnel present at a military installation. Pontiac fever has an incubation time of 24-72 hours and an attack rate of >90%. Hospitalization for Pontiac fever is uncommon, and the case-fatality rate is 0% (CDC). Like Legionnaires’ disease, Pontiac Fever is not transmitted from person to person.
Jjemba et al. found that opportunistic pathogens, particularly Legionella, had potential for regrowth in the distribution portion of water reuse systems under certain conditions. Since shower water is heated before use, and would retain some of this heat for a period of time after use at a temperature ideal for Legionella growth, Legionella growth in water for showering is of particular concern for a shower water reuse system at military installations. Table 2 shows the basics of the Legionella parasite.
Table 2. Legionella basics
Exposure Assessment
Exposure Scenario
We conducted an exposure assessment for using treated Class II water for showering at a military encampment. Figure 1 presents the exposure pathway model for risk assessment.
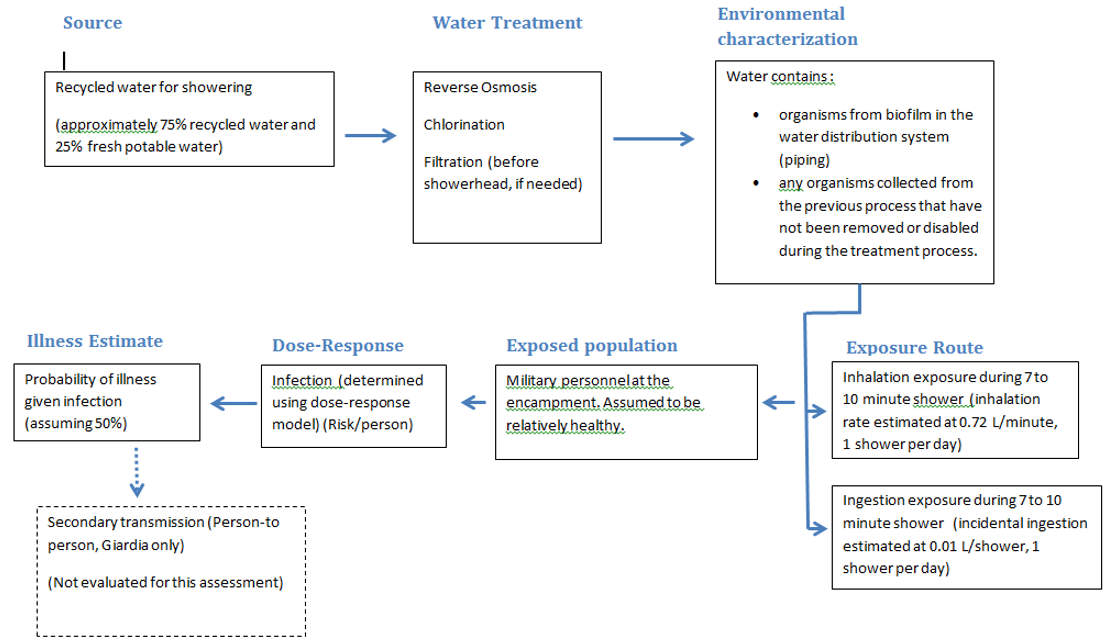
Figure 1. Exposure pathway model for risk assessment at a military encampment
Pathway # 1: Exposure to Legionella via the inhalation of shower aerosols containing biofilm-associated Legionella detached from in-premise plumbing
- First, the biofilm-associated Legionella multiplies within the biofilm or within a population of infected protozoan hosts within the biofilm of the in-premise plumbing. During a showering event, the biofilm-associated Legionella detaches from the biofilm into the bulk water. The Legionella is then aerosolized after flowing through the shower head with a portion of the aerosolized bacteria in aerosols of a respirable size. Once inhaled, processes within the lungs result in only a fraction of the inhaled bacteria reaching the alveolar region; the deposited dose (Shoen). A dataset for L. pneumophila concentrations found in greywater was compiled from a literature review. The L. pneumophila concentration dataset was fit to a log-normal distribution using Oracle® Crystal Ball.
Pathway # 2: Exposure to Giardia via incidental ingestion of water while showering
- A portion of the Giardia cysts that remain in water can cause infection if they reach the stomach while passing through mouth, throat and esophagus after ingestion. Excystation of the Giardia cysts to the trophozoite form is initiated in the stomach and completed in the small intestine. The trophozoites divide, attach to the small intestine, and then detach for unknown reasons. During the encystment process, they become a rounded and elaborate cyst wall that protects the cyst as it is excreted and carried through water and other media (CDC). A dataset for Giardia concentrations found in greywater was compiled from a literature review. The concentration data was then fit in R software and resulted in a normal distribution with a mean of 1.29 and a standard deviation of 0.6.
For both pathways, we modeled the behaviour of Legionella and Giardia through the shower water using a linear progression: Sources of Class I water → Uses of Class I water → In base water treatment plant → Shower. Our algorithm for exposure depends on the pathogen assessed. For Giardia we considered the duration of a shower and the water flow rate. Another important parameter is the ingestion rate during a shower. Finally, we used the partition coefficient between water and air was used to assess how much water is ingested. For Legionella there were more parameters involved, because Legionella is inhaled and in the lungs had to be modelled. Duration of a shower and water flow rate are the basic parameters. Then the percent of Legionella that is retained in the lungs, the inhalation rate, and partition coefficient between water and air, and all episodes that occur during a shower were used.
Dose Response
For our risk assessment of Giardia infection during showering, we used the dose-response model for the Giardia duodenalis found on the Quantitative Microbial Risk Assessment wiki website (QMRAwiki) (Enger). The optimized model was based on feeding studies conducted by Rendtorff on adult male prisoners. The conclusion from this study was supported by further evaluation (Rose et al.; Zmirou-Navier et al.).
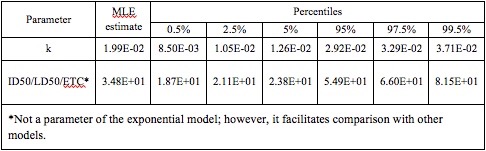
The best fit for the dose response model was exponential, with median k = 1.99E-02 and 95% = 2.92E-02. The percentiles resulting from 10,000 bootstrap iterations are shown Table 3, as they appear on the QMRA wiki site
Table 3. Optimized k parameter for the exponential model, from 10,000 bootstrap iterations
The dose-response model for Legionella pneumophila was also obtained from the website QMRA wiki (Weir). The optimized model, based on experiments from Muller 1983, involved exposure of guinea pigs to Legionella via the inhalation route in a modified aerosol infection chamber.
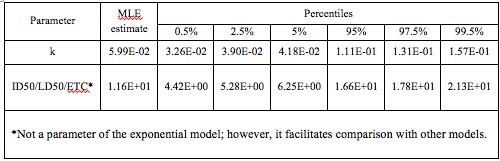
The best fit for data from Muller was an exponential model, with an optimized k parameter of 5.99E -02 and an endpoint of infection. The percentiles resulting from 10,000 bootstrap iterations are shown in Table 4, as they appear on the QMRA wiki (Weir).
Table 4. Optimized k parameter for the exponential model, from 10,000 bootstrap iterations
Risk Characterization
When running this model, the primary consideration taken into account is the safety of the U.S. soldiers and other military personnel who are taking showers with the treated Class II wastewater. In this model, the primarily concern is with safety as it pertains to the microbial contaminants Giardia and Legionella. The U.S. military states that the suggested risk level for gastrointestinal illness is 0.5%, and the suggested risk level for respiratory illness is 0.4% (Rose). These risk levels will be compared to the risks simulated herein. If the treated Class II water does not conform to the above mentioned risk levels, additional treatment methods, such as ultra-filtration and UV light, will be suggested for the U.S. military to implement in order to make the treated Class II wastewater safe for shower reuse. However, if it does not seem plausible to have safe Class II wastewater after conducting the risk characterization, then it will be suggested that the U.S. military not switch from its current potable water sources for showering.
Risk Model
Legionella infects people by inhalation, which in our study translates to breathing the Legionella-containing aerosols during a shower event. In a hypothetical model for the inhalation of Legionella during a shower event, first, the biofilm-associated Legionella will propagate within the biofilm inside the premise plumbing. Then, during a shower, these Legionella detach from the biofilm and slough off into the feed water. Next, the Legionella-containing water is sprayed out of the shower head and aerosolized to a number of sizes, a portion of which are of respirable size. When the person taking the shower breathes during a shower, a fraction of the respirable-sized Legionella droplets deposit in the lungs. Eventually, a fraction of such Legionella will result in illness of the person, leading to incapacity to execute the mission.
Giardia infects people by ingestion, and in this case, it pertains to the accidental ingestion of Giardia-containing shower water. For a hypothetical model of ingestion of Giardia during a shower event, the remaining Giardia in the feedwater get released out of the showerhead, and the fraction that is retained in the water will accidentally be ingested by the person. A portion of these Giardia will cause infection of the people, which may result in illness and inability to execute the mission.
A risk assessment was performed to evaluate the risk of showering with treated Class II water at a forward (deployed) military base. The water treatment methods considered in our scenario are based on treatment methods currently known to be in use at military encampments. According to the Surface Water Treatment Rule (EPA), a minimum of 3 log10 of Giardia removal and a minimum of 4 log10 of viruses removal are mandated. One of the existing technologies available in the Army is reverse osmosis (RO), which can give us more than 3 log10 of Giardia removal and 3 log10 of virus removal (EPA). Therefore, the Giardia removal solely by RO has met the requirements, and since viruses are much smaller than Legionella, 3 log10 of virus removal is a conservative estimation for Legionella removal. Furthermore, although there is no limit for Legionella concentration in water, EPA believes that if Giardia and viruses are removed or inactivated according to the treatment techniques in the Surface Water Treatment Rule, Legionella will also be controlled. Therefore, the downstream disinfection method only needs to focus on 1 more log10 removal of virus or Giardia, rather than directly focus on Legionella removal. The greywater is first treated with a RO system, with an estimated greater than 3 log10 removal of Giardia, rather than directly focus on Legionella removal.
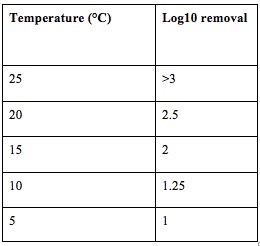
As discussed in the US army document “Non-potable water substitution” from the U.S. Army Center for Health Promotion and Preventive Medicine (USACHPPM), “the goal of disinfection is to produce a detectable chlorine residual in the source water 30 minutes after chlorine addition”. Adding chlorine is a feasible option to reduce greywater pathogenic contamination. Because excessive chlorine may contribute to disinfection byproducts production, a smallest CT value that can satisfy 1 log10 removal of Giardia from the temperature ranging of 5 – 25° C was chosen (Table 5).
Table 5. Temperature Treatments and Resulting Log10 Removal of Giardia or Legionella by the chlorination step
Legionella re-growth in the premise plumbing and water heating system is common (Brazeau and Edwards). Systems that maintain a consistent inflow of water from the main distribution line tend to have continuous levels of disinfectant. However, as water remains stagnant in the system, such as the systems found in water heaters, disinfectants will decay and water quality will decrease (Brazeau and Edwards). It has been reported that “use of an electric water heater was the most significant factor leading to Legionella contamination in hot water in the home” (Lévesque et al.).
The optimal temperature for Legionella proliferation in water varies between 32°C and 35°C, but it can easily proliferate at temperatures up to 45°C. Usually, there is no growth above 55°C, and a temperature of over 60°C has a bactericidal effect. Thus, the World Health Organization recommends that water is heated and stored at 60°C (Lévesque et al.). The risk of contamination is practically nonexistent for these heaters set at 60°C. According to some literature, it is advisable to set water heaters at 60°C and they should all be equipped with anti-scald devices to deliver water at around 49°C to the entire household.
The estimated risks of infection and illness for the aformentioned scenarios are described in Tables 6 and 7.
Table 6. Giardia Best Estimates of Risk
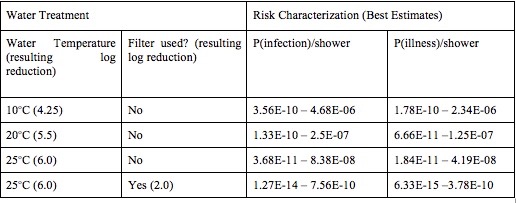
Table 7. Legionella Best Estimates of Risk
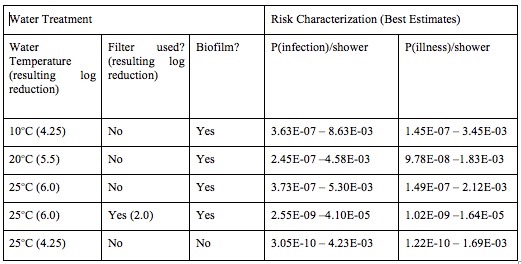
In the risk assessment for Legionella, the presence of an additional filter before the shower head, which reduced the Legionella concentration by 2 Log10, resulted in the very low overall risk prediction for illness (about 1x 10 -7). Similarly, if we assume that the treatment removes biofilm from the system, the risk of illness is very low (about 4 x 10-7).
Even without the assumption of additional filtration, however, and the assumption of only a 4.25 Log10 reduction with use of RO and chlorine, the median risk of infection from Legionella appears to be acceptable low for this population (about 6.35 x 10-6), as shown in Figure 2.
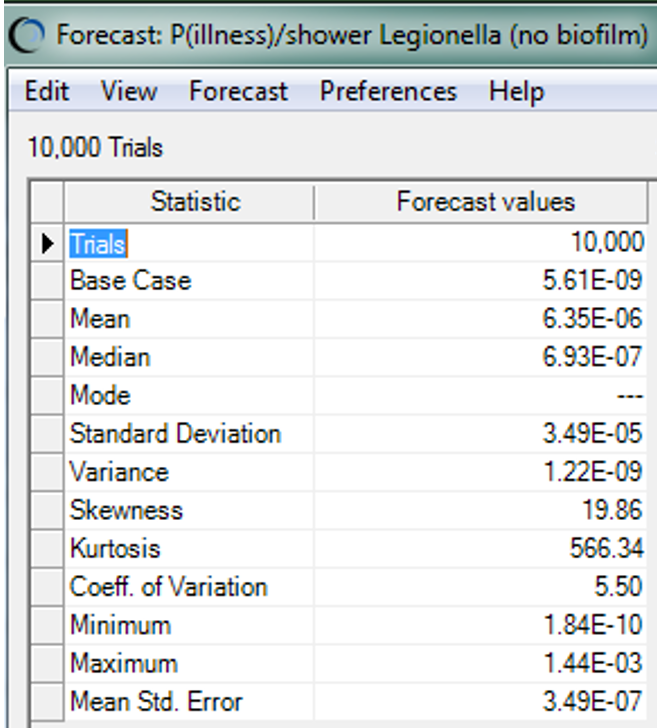
Figure 2: Summary statistics for the risk of illness from inhaling Legionella in a showering scenario
Sensitivity Analyses
It was observed that the initial concentration of Giardia in the untreated greywater contributes to more than 90% of the total variance, followed by the flow rate of the shower. The contribution to variance by the duration of the shower is consistently comparable to that of the flow rate of the shower. Water treatment (a sequence of reverse osmosis followed by free chlorine addition) has the most significant impact on the risks that soldiers may be exposed to. The greater the log reduction of Giardia, the less risks soldiers may encounter during a showering event, shown in Figure 3.
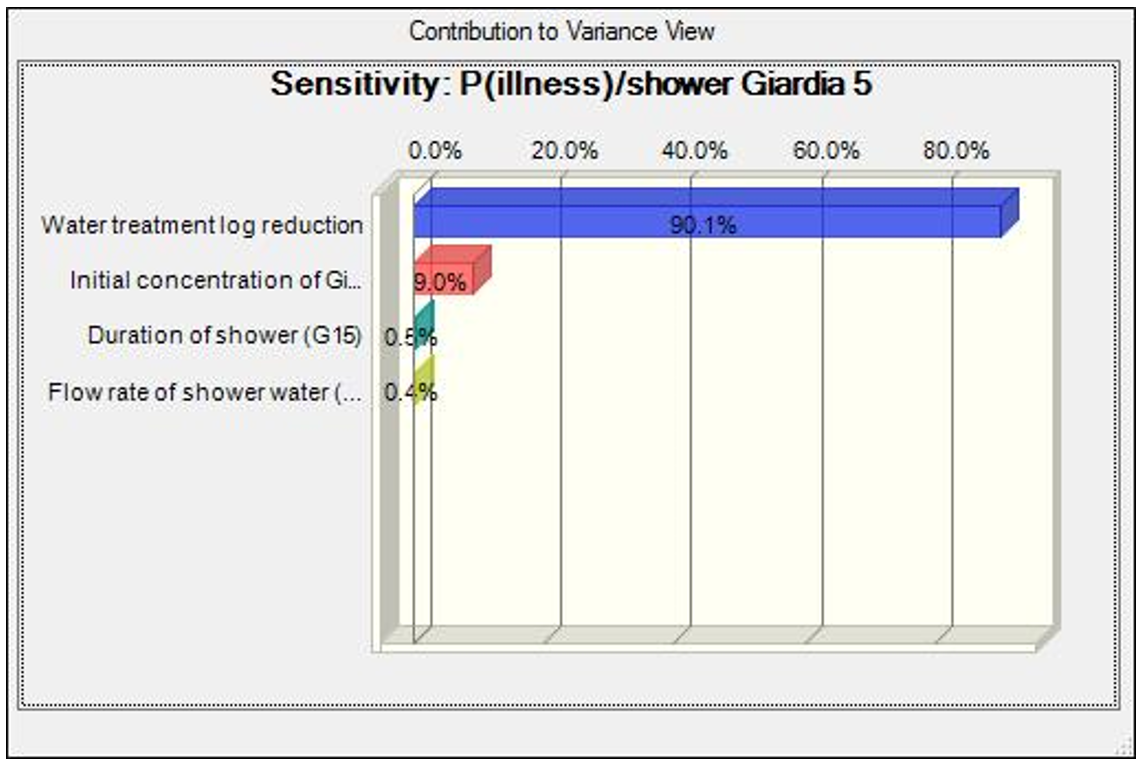
Figure 3. Sensitivity analysis for the risk of illness from ingesting Giardia in a showering scenario
For the Legionella risk assessment, it was observed that the detachment rate of Legionella in the untreated greywater contributes to more than 97% of the total variance, followed by the duration of the shower. The contribution to variance by the duration of the shower is consistently comparable to that of the flow rate of the shower, except when biofilm is not considered in the model. Water treatment, a sequence of reverse osmosis followed by addition of free chlorine in this system, has significant impact on the risks to soldiers of exposure to Legionella. More effective is the control of biofilm. The model shows that when no biofilm is present, the most significant factor is the initial Legionella concentration, rather than the detachment rate of Legionella in biofilm.
In order to reduce uncertainties in these inputs, we propose that more data be found and a systematic evaluation of the distribution of the data be done. As more data points are found, the distribution of the data set will be closer to the true distribution of the whole population. In addition, increasing the amount of Monte Carlo sampling iterations will also help in reducing the uncertainties.
Limitations
People over 50 years old and people with weak or compromised immune systems are more susceptible to Giardia and Legionella. For Legionella specifically, when biofilm is present, the risk of infection, and resulting illness, is driven by the detachment of the biofilm. If no additional filtration is used before the showerhead, the biofilm could potentially increase the risk to those who are more susceptible to Legionnaires’ disease. However, in this population of military personnel, most of the population is expected to be relatively healthy. Nonetheless, all personnel could be susceptible to Legionella exposure leading to Pontiac fever.
Overall, at higher levels of treatment (higher Log10 reductions) in Giardia, the reduction in risks given the same amount of reduction in Giardia (the most influential parameter for infection caused by Giardia) reduces the risk of infection by one order of magnitude more than the reduction in risk of infection by Legionella. This is due to the identical Log10 reduction exerted on Legionella or Giardia. As the Log10 reduction of the treatment train increased, the 90% percentile of the risk decreased significantly by orders of magnitude. For Legionella, in general the 90% percentile of the risk decreased significantly by increasing the Log10 reduction of the treatment train from 4.25 to 5.5 Log10.
Ideally, the assumptions made with the model could be tested by collecting data from real-world systems. In lieu of data from a system in use, a laboratory model to test the assumptions would help to better understand if the parameters of the model hold true. A third option is to use data from the literature to test assumptions. An additional option is to test the model through collection of epidemiologic data on the people ill (showing symptoms) and doing a comparison with the model prediction.
Risk Management & Communications
Acceptable health risks for water reuse in the military may be variable when compared to regulatory standards for the general public or U.S. citizens. The acceptable risk of infection from Giardia and Legionella is contingent on the specific base or area of operations, military personnel using the water, the surrounding community, and the mission being carried out. While the results of this assessment suggest that using class II water is a useful method to reduce water consumption, the continued use of potable water for showering may be necessary for certain military operations. When determining an acceptable value for class II water usage, health risk considerations, tradeoffs between benefits and costs, and economic considerations should be used to determine acceptability on a case by case basis. Using only gray water, a mixture of greywater and potable water, additional water filtration methods, biological treatments, and increasing chlorine use should be used to incorporate an acceptable level of Giardia and Legionella in the water source. Additionally, decreasing shower duration and frequency will lower risk of infection, as shown by the models. Based on a literature review, this risk assessment used an acceptable risk level of 0.01% of infection of Giardia and Legionella. Each water treatment log reduction resulted in a lower probability of infection/shower and the probability of illness/shower. The highest simulated probability of illness/shower (Giardia) was 1.00E-06, and the lowest simulated probability of illness was 1.18E-10 after increased filter reduction in the shower head. None of the scenarios simulated concentrations above or close to 0.01% infection. Legionella simulation results, as displayed above, were all well below 0.01% probability of infection.
This risk assessment suggests that using gray water for showering may be a productive method to conserve water at military bases while posing minimal health risks to soldiers. Costs to filter and reuse the water to an acceptable level will be dependent on the specific military base and operations of the soldiers. The goal of the risk communication strategy in this risk assessment is to share the range of information for each for each scenario, and the associated risks of showering with reused water. The simulation outputs should be shared with the commanding officers, soldiers, and surrounding community to make informed and cost effective decisions.
U.S. Departments of the Army, Navy and Air Force. 2010. Technical Bulletin. Sanitary Control and Surveillance of Field and Water Supplies. TB MED 577/ NavMED P- 5010-10/AFMAN 48-138_IP. Retrieved from http://armypubs.army.mil/med/DR_pubs/dr_a/pdf/tbmed577.pdf/ Accessed August 5, 2015.
References
-
Defense, U. D. of. (2013). Army Net Zero: Energy Roadmap and Program Summary. Retrieved from https://www.asaie.army.mil/Public/ES/netzero/docs/FY13%20Army%20Net%20Zero%20and%20Energy%20Program%20Summary.pdf
-
Blanky, M. ., Rodríguez-Martínez, S. ., Halpern, M. ., & Friedler, E. . (2015). Legionella pneumophila: from potable water to treated greywater; quantification and removal during treatment. Science of the Total Environment, 533, 557–565. https://doi.org/https://doi.org/10.1016/j.scitotenv.2015.06.121
-
Birks, R. ., Colbourne, J. ., Hills, S. ., & Hobson, R. . (2004). Microbiological water quality in a large in-building, water recycling facility. Water Science and Technology, 50, 165–172. Retrieved from https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.484.957&rep=rep1&type=pdf
-
Birks, R. ., & Hills, S. . (2007). Characterisation of indicator organisms and pathogens in domestic greywater for recycling. Environmental Monitoring and Assessment, 129, 61. Retrieved from https://link.springer.com/article/10.1007/s10661-006-9427-y
-
Friedler, E. ., Yardeni, A. ., Gilboa, Y. ., & Alfiya, Y. . (2011). Disinfection of greywater effluent and regrowth potential of selected bacteria. Water Science and Technology, 63, 931–940. https://doi.org/https://doi.org/10.2166/wst.2011.272
-
Leoni, E. ., Legnani, P. ., Sabattini, M. B., & Righi, F. . (2001). Prevalence of Legionella spp. in swimming pool environment. Water Research, 35, 3749–3753. https://doi.org/https://doi.org/10.1016/S0043-1354(01)00075-6
-
Rose, J. B. (2000). Future health assessment and risk-management integration for infectious diseases and biological weapons for deployed US forces. In Strategies to Protect the Health of Deployed: Assessing Health Risks Deployed US Forces (pp. 59–112). Retrieved from https://nap.nationalacademies.org/read/9709/chapter/6
-
Painter, J. E., Hlavsa, M. C., Collier, S. A., Xiao, L. ., Yoder, J. S., & Gargano, J. W. (2015). Cryptosporidiosis surveillance–United States, 2011-2012; Giardiasis surveillance–United States, 2011-2012. Morbidity and Mortality Weekly Report: Surveillance Summaries, 64(3). Retrieved from https://www.jstor.org/stable/24806287
-
Prevention, C. for D. C. (2015). Parasites - Giardia: Pathogens & Environment. Retrieved from http://www.cdc.gov/parasites/giardia/pathogen.html
-
Prevention, C. for D. C. (2015). Parasites - Giardia: Sources of Infection & Risk Factors. Retrieved from http://www.cdc.gov/parasites/giardia/infection-sources.html
-
Prevention, C. for D. C. (2015). Parasites- Giardia General Information. Retrieved from http://www.cdc.gov/parasites/giardia/general-info.html
-
Prevention, C. for D. C. (2015). Parasites - Giardia: Treatment.
-
Health, M. D. of. (2015). About Legionellosis (Legionella). Retrieved from https://www.health.state.mn.us/diseases/legionellosis/basics.html
-
Health, M. D. of. (2015). About Pontiac Fever. Retrieved from https://www.health.state.mn.us/diseases/legionellosis/pontiac.html
-
Prevention, C. for D. C. (2015). Legionella: Legionnaires’ Disease and Pontiac Fever. Retrieved from http://www.cdc.gov/legionella/clinicians.html
-
Jjemba, P. K., Weinrich, L. A., Cheng, W. ., Giraldo, E. ., & LeChevallier, M. W. (2010). Regrowth of potential opportunistic pathogens and algae in reclaimed-water distribution systems. Applied and Environmental Microbiology, 76, 4169–4178. https://doi.org/10.1128/AEM.03147-09
-
Schoen, M. E., & Ashbolt, N. J. (2011). An in-premise model for Legionella exposure during showering events. Water Research, 45, 5826–5836. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0043135411004684?via%3Dihub
-
Enger, K. S. (2013). Giardia duodenalis: Dose Response Models. Retrieved from http://qmrawiki.canr.msu.edu/index.php/Giardia_duodenalis:_Dose_Response_Models
-
Rendtorff, R. C. (1954). The experimental transmission of human intestinal protozoan parasites. I. Endamoeba coli cysts given in capsules. American Journal of Hygiene, 59, 2. https://doi.org/https://doi.org/10.1093/oxfordjournals.aje.a119633
-
Rose, J. ., Haas, C. ., & Regli, S. . (1991). Risk assessment and control of waterborne giardiasis. American Journal of Public Health, 81(6), 709–713. https://doi.org/10.2105/ajph.81.6.709
-
Zmirou-Navier, D. ., Gofti-Laroche, L. ., & Hartemann, P. . (2006). Waterborne microbial risk assessment: a population-based dose-response function for Giardia spp.(E. MI. RA study). BMC Public Health, 6. https://doi.org/10.1186/1471-2458-6-122
-
Weir, M. H. (2017). Legionella pneumophila: Dose Response Models. Retrieved from http://qmrawiki.canr.msu.edu/index.php/Legionella_pneumophila:_Dose_Response_Models
-
Muller, D. ., Edwards, M. L., & Smith, D. W. (1983). Changes in Iron and Transferrin Levels and Body Temperature in Experimental Airborne Legionellosis. The Journal of Infectious Diseases, 147, 2. https://doi.org/https://doi.org/10.1093/infdis/147.2.302
-
Agency, U. E. P. (2015). Promoting Sustainability and Resiliency Through Net Zero and Net Positive Technologies and Approaches. Retrieved from https://www.epa.gov/sustainability
-
Medicine, U. A. C. for H. P. and P. (2010). Emergency Drinking Water Disinfection Procedure.
-
Brazeau, R. H., & Edwards, M. A. (2011). A review of the sustainability of residential hot water infrastructure: Public health, environmental impacts, and consumer drivers. College Publishing, 6, 77–95. https://doi.org/10.3992/jgb.6.4.77
-
Lévesque, B. ., Lavoie, M. ., & Joly, J. . (2004). Residential water heater temperature: 49 or 60 degrees Celsius?. Canadian Journal of Infectious Diseases and Medical Microbiology, 15, 11–12. https://doi.org/10.1155/2004/109051