Authors: Borel, K., Daley, K., Gonzalez, R., Henderson, M., Robson, A.
Problem Statement
The United States Army has forward operating bases (FOB) around the world which are home to approximately 13,650 military personnel deployed to combat zones, according to BBC , Dempsey , and DOD . Providing potable water to FOBs typically involves transporting it long distances resulting in large costs and reduced capacity, since between 70-80% of convoy capacity is occupied by water and fuel transport, according to Moore and Noblis . A proportion of this potable water is used for non-drinking water uses such as vehicle washing and personal care. Reusing greywater, wastewater from food preparation, laundry and showering, is therefore desirable to reduce the volume of imported water, its associated cost and reduced capacity associated with the provision of potable water to FOBs.
Greywater typically contains a moderate number of pathogens which may explain the increased reports of skin rashes with greywater reuse on FOBs. The U.S. Army Public Health Command (USAPHC ) conducted a quantitative microbial risk assessment (QMRA) assessing the risk from pathogens in greywater to personnel on FOBs in 2014. This QMRA identified a number of pathogen exposure pathways for FOB populations including showering, food production, laundry, toilet flushing, vehicle washing and dust suppression. This initial QMRA, limited to the risks posed by E.coli due to in-field assay limitations, identified ingestion whilst showering as a major exposure route, making up 75% of all greywater reuse. according to Good . Further QMRAs using different pathogens are required to better characterize the risk of infection and illness from different pathogens and exposure routes. New QMRAs will introduce new treatment options for greywater to be evaluated. Pseudomonas aeruginosa (P. aeruginosa) was identified as an important reference pathogen for future assessments.
Stakeholders
A number of stakeholders are involved in this problem. The potential victims put at risk through dermal contact with P. aeruginosa include all personnel showering on a FOB. The potential victims include:
- Military personnel
- Non-military contractors
- Indigenous populations on base (Dismiss as not going to shower on base)
The stakeholders able to fix or exacerbate this problem include those with the power to make decisions, budget holders, personnel involved in treating symptoms of illness and personnel able to treat greywater including:
- Federal government
- Commanding officers at FOBs
- Healthcare workers
- Engineers and Scientists
Problem Question
Following USAPHC recommendations the scope of this assessment will focus on the risk to deployed personnel living on a FOB from P. aeruginosa through dermal contact from showering. What intervention is needed to reduce risk of illness from P. aeruginosa to a level acceptable to the US army?
Hazard Identification
P. aeruginosa is a gram-negative, rod-shaped, facultative aerobe. P. aeruginosa is ubiquitous in the environment, living on inert and living (i.e. human and plant tissue) matrices, according to Botzenhart and Doring . The ability to use a variety of nutrients has made this versatility possible (Botzenhart and Doring) . Even though found throughout the environment P. aeruginosa is an emerging opportunistic pathogen of clinical significance.
Life stages
In environmental matrices P. aeruginosa could be found in a planktonic state (very motile), in biofilms, or surface-attached. P. aeruginosa biofilms are found in a variety of surfaces including metals, plastics, and tissue (Bedard et al., 2016) . Biofilm formation is thought to be a survival mechanism for P. aeruginosa but can have positive or negative effects on the environment (e.g. medical device, premise-plumbing, aquatic environment according to Bedard et al.). Having a single, polar flagellum and pili are important for P. aeruginosa survival—aiding in attachment to cell surfaces (including host cell adherence), structure, and mobility (active movement and chemotaxis, according to van Delan ).
Mode of transmission
P. aeruginosa has numerous reservoirs due to their ubiquitous nature, allowing for transmission via food, water, air, and fomites, according to Botzenhart and Doring. Common infections in hospital settings are through fomite transition (e.g. faucets, medical devices), food ingestion, and by health care workers (person to person). Numerous infections at recreational swimming pools and hot-tubs occur through skin immersion, aerosolization (e.g. steam, aerosolized droplets), and ingestion.
Epidemiological information
P. aeruginosa is primarily a nosocomial (hospital-associated) pathogen. P. aeruginosa infections account for approximately 10% percent of all hospital-associated infections, making up 17% of nosocomial pneumonia, 7% of urinary tract infection, and 8% of surgical-site infection, according to Dembry et al. and NNIS.
Outbreaks of non-hospital related P. aeruginosa infections occur in water-related recreational settings, according to Mena and Gerba. Folliculitis (inflammation of hair follicles) and skin rashes has been documented after shower/bath, heated swimming pool, whirlpool, and hot-tub exposure, according to Rice et al.. Infection after shower/bath exposure has only a few documented cases, but most likely underestimated (Zichichi et al., 2000) . P. aeruginosa is one of the more frequently isolated pathogens in recreational pools and hot-tubs (Rice et al.). Ear and skin infections are the dominant concerns associated with P. aeruginosa recreational pools and waters, according to Hajjartabar and Washburn et al. .
Clinical information
P. aeruginosa infections can involve any part of the body. Infections commonly affect the respiratory tract, central nervous system, ear, eyes, bones and joints, gastrointestinal tract, urinary tract infection, skin and soft tissue, blood, and heart, according to Mena and Gerba. The infectious dose of P. aeruginosa is unknown due to the multiple transmission routes and susceptibility of immunocompromised population. Patients with severe burns, cancer, AIDS, and cystic fibrosis are especially at risk (Botzenhart and Doring ). While most infections are treatable, increasing antimicrobial resistance of P. aeruginosa is concerning, according to CDC.
The focus of this QMRA is on skin infections. Epidermal infections often result from P. aeruginosa contact superficially or infiltrating through the skin at the site of an open wound. In superficial cases, the rash heals in less than 10 days. Following severe skin damage, infection can be promoted to a deeper bacterial infection, according to Rice et al. . Within 48 hours of exposure itchy urticarial papulas and deep pustules may develop, according to Molina et al. . Acne, malaise, fatigue, fever or headache can accompany the rash.
Detection methods
P. aeruginosa can be detected by culture- and molecular-based methods in environmental and clinical settings. Rapid methods have been developed and used to identify P. aeruginosa in water samples, according to Khan and Cerniglia . However the combination of culture-based presumptive identification, confirmed by molecular methods is necessary for clinical diagnosis in immunocompromised people. Depending on the infection, laboratory studies may include complete blood counts, blood cultures, urinalysis, gram stains.
Exposure Assessment
This exposure assessment frames the potential wastewater reuse scenario being assessed. The factors necessary to characterize the exposure of interest and assumptions made are identified. As stated in the initial assessment conducted by the USAPHC, the complete information necessary to characterize the full potential of exposure is not available. Due to these data limitations, only a small subset of exposure factors values were necessary to conduct an exposure assessment.
Exposure Scenario
A range of potential exposure scenarios were identified in the previously conducted QMRA, according to USAPH . Of those scenarios, the most likely cause of skin rashes is from dermal contact with P.aeruginosa via showering since it involves full bodily contact with greywater. For this reason this exposure scenario was considered the most important to evaluate. The exposure population being considered is military personnel only as other populations present on FOBs such as indigenous populations are unlikely to take showers on site. While non-military contractors living on FOBs are also using shower facilities, it is assumed that they are similar to military personnel in age range and health and can be considered military personnel for this assessment. It is assumed that all military personnel are healthy individuals ages 18-60.
Exposure Pathway
Greywater that has been categorized as suitable for unrestricted non-potable reuse activities is used for showering. As depicted in Figure 1, greywater is piped from a centralized holding tank to a shower system.
Figure 1. Pictorial model of potential exposure pathway of greywater transport to FOB showers.
The army has multiple shower systems available, however the containerized shower system is typically used on FOBs. The containerized shower system (Figure 2) is a standalone system that provides a 10-minute shower at a flow rate of 2.5 gallons (9.5 liters) per minute resulting in 25 gallons (95 liters) of water use per shower, according to DA and USAPHC . The containerized shower system is capable of providing showers to 150 personnel on a continual basis and includes a 3,000 gallon (11,356 liters) water tank, according to ILSC NATICK .
Figure 2. Containerized shower system schematic (Figure from: ILSC NATICK )
The exposure route being considered is direct dermal contact. Although inhalation and unintentional ingestion pathogens are also possible during showering, they are not considered in this exposure scenario as dermal contact is the primary route leading to P. aeruginosa related skin infections, according to Mena and Gerba . The shower exposure pathway is highlighted in yellow in the conceptual model below (Figure 3).
Figure 3. Conceptual model of the exposure pathway for P. aeruginosa for water reuse.
Exposure Factors
Concentration values of P. aeruginosa in FOB greywater holding tanks are not available. Similarly, detailed information about distribution pipe material has not been documented and likely varies greatly between FOBs, which eliminates the possibility of determining relevant regrowth and biofilm factors. In the absence of actual concentration data, representative values based on levels of P. aeruginosa in household greywater tanks were used, according to Casanova et al. and Winward . The full concentration was used as a dose. The USAPHC compared swimming and showering exposure data and determined that the two activities are similar enough to make the dose-response from one activity applicable to the other. A time correction factor was added to account for the difference in duration of exposure. The specific values used are described in the following section on dose-response.
Acceptable Risk
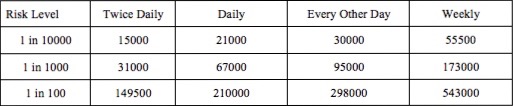
An acceptable level of risk must be determined in order to set a microbiological limit. In this context risk is the probability of skin rash symptoms in the population following exposure to greywater. Determining an acceptable level of risk for skin rashes is particularly difficult due to the range of skin conditions and their disease outcomes, according to Hay et al. . In civilian life, 10-6 or 1 in a million chance of developing a specific disease is often used in environmental impact assessments is endpoint as an acceptable level of risk. A previous risk assessment (USAPHC ) of gastrointestinal illness from greywater reuse noted that during deployment, to an FOB for example, environmental health risks should be reduced as low as practicable, which is generally accepted as the equivalent U.S. civilian standards. However, in FOB contexts, mission readiness and/or the ability to complete a mission are likely to alter the acceptable level of some risks. Furthermore, there are currently no U.S. civilian standards for wastewater reuse for showering. Inline with the previous assessment, (USAPHC ) three levels of acceptable risk are presented, allowing the FOB officer in charge (OIC) to maintain flexibility in their ongoing risk assessment. The three daily risk levels are:
- 1 in 100 (10-2), whereby on average 1 person in every 100 will develop a skin rash from P.aeruginosa if they follow a defined shower regime and the P.aeruginosa concentration is at this risk level.
- 1 in 1,000 (10-3), whereby on average 1 person in every 1,000 will develop a skin rash from P.aeruginosa if they follow a defined shower regime and the P.aeruginosa concentration is at this risk level.
- 1 in 10,000 (10-4), whereby on average 1 person in every 10,000 will develop a skin rash from P.aeruginosa if they follow a defined shower regime and the P.aeruginosa concentration is at this risk level.
The shower regimes are defined as:
- Daily - Each person showers once in a 24 hour period.
- Twice Daily - Each person showers twice in a 24 hour period.
- Every Other Day - Each person showers once in a 48 hour period.
- Weekly - Each person showers once in a one week (168 hour) period.
Dose Response
The dose response model from Roser et al. (Equation 1) was developed for P. aeruginosa in pools for dermal infection.
Pinf = 1 - e(-r*C*f) (1)
where, Pinf is the probability of infection, f is the time correction factor, C is the density of the pathogen (c.f.u./ml), and r is the constant 4·3 × 10−7 c.f.u./ml P. aeruginosa. These model distributionwere sampled 500,000 times in a monte-carlo simulation using Oracle Crystal Ball plug-in for Microsoft Excel. When applying the correction factor for the biofilm and growth, Equation 2 below was used:
Pinf = 1 - e(-r*(C.Cr).*f) (2)
where, Cr = 10(Y), Y = the log of the correction factor and other quantities as in equation (1). Note, the correction factor is applied after sub-sampling the triangular distribution for concentration.
The dose response model was used to model risk. It was assumed that there was a 1 to 1 correlation between the probability of the risk of infection to the probability risk of illness.
Risk Characterization
The dose-response model identified the risk to personnel showering once per day. However, showering regimes are likely to vary depending on the roles of individuals on FOBs and the function and situation of the FOB. Point estimates of risk for individuals using different shower regimes are presented in Table 1.
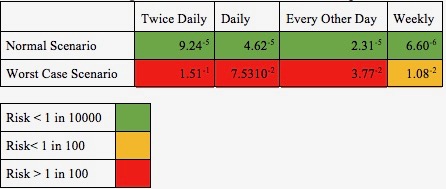
Table 1. Risk of P. Aeruginosa infection with different shower regimes.
During the normal scenario, risk was consistently below the 1 in 10,000 acceptable level. In the worst case scenario, risks consistently exceeded the 1 in 100 acceptable level when showering more often than every other day (Table 1).
The risk assessment considered the FOB population to be equally susceptible to P. aeruginosa infection. This is unlikely to be the case. FOB personnel often operate in harsh environments performing manual tasks, increasing their susceptibility to infection due to skin damage through grazes or UV damage for example.
Concentrations to achieve acceptable risk levels
The model was used to predict risk values at varying concentrations to give a point concentration of P. aerguniosa required to achieve each acceptable risk level for each shower routine (Fig. 4). Figure 4 shows the P. aeruginosa concentration limit required to achieve each risk level for personnel using each shower regime. If a limit of 3 x 104 CFU/100ml is maintained, then all personnel using the showers would achieve a risk level of 1 in 1000, and those showering every other day or weekly would achieve a risk level of 1 in 10,000. This therefore appears to be a sensible recommended concentration for P. aeruginosa in greywater reused for showering.
Figure 4. Risk against log of concentration of P. aeruginosa in greywater.
Table 2. Maximum concentrations (CFU/100mL) of P.aeruginosa in greywater reused for showering which will still achieve each risk level.
This QMRA assessment was performed following reports of skin rashes with greywater reuse on FOBs. The normal scenario, which does not take into account biofilm sloughing and bacterial growth, suggests that the risks are likely to be less than 1 in 10,000 for personnel showering daily. This suggests that P. aeruginosa are an unlikely cause of skin rashes, particularly if the prevelance of the rashes is much greater than 1 in 100 personnel, since this is around the value of the ‘worst case’ scenario. A better characterisation of the greywater produced on FOBs is required to determine both the concentration of P. aeruginosa, and to identify other substances or microbes which may be responsible for the skin rashes.
Based on the dose-response model, we propose three levels of risk to allow flexibility in OIC of FOBs maintaining mission readiness and mission performance. Since personnel on an FOB are likely to have different shower regimes, this was taken into account when predicting risks (Table 4). It was evident that maintaining a concentration below 3 x 104 CFU/100ml would allow all showering regimes to be below the 1:1000 risk threshold and those showering less often than daily to achieve the 1:10,000 threshold. As a generic guideline across all FOBs it is recommended that a concentration of 3 x 104 CFU/100ml when measured at point of use is maintained.
A sensitivity analysis was performed on the output of the monte-carlo simulation (Figure 5).
Figure 5. Sensitivity analysis
Figure 5 indicates that the uncertainty in r (dose-response parameter) and in the concentration of P. Aeruginosa have almost equal effect on risk. However, when applied, the large range in the biofilm correction factor had a large impact on the value of risk. This presents a large limitation. The biofilm correction factor was assumed from studies who also reported large variability in this factor, according to Lautenschlager et al. , this highlights the need for better monitoring and modelling of biofilm systems, particularly that of P. aeruginosa.
Risk Management & Communication
Below are a list of recommendation for risk reduction:
- Disinfection and/or Secondary treatment of greywater for a large log reduction of pathogens in graywater, according to Rice et al. .
- Medical Treatment: Pires et al. states that antibiotics and phage therapy are medical treatment for P. aeruginosa. Use of phage therapy uses in vivo and in vitro phage therapy for patients with P. aeruginosa infections.
- Rice et al. states that tertiary water treatment to reduce organic loading. P. aeruginosa is able to grow from trace amounts of amounts of carbon and warm temperatures. Graywater is an ideal environment for growth of P. aeruginosa because of the conditions present in holding tanks.
- Post-outbreak monitoring of skin rash occurrences, according to Rice et al.. Monitoring outbreaks of skin rashes and cluster occurrences of infection will aid in identifying problems and reducing the risk.
- Characterization of graywater: The actual concentration of P. aeruginosa in the graywater at FOBs is not known. Use of microbiological methods for detecting the pathogen would improve the dose response model.
- Flash Water Heater: Point of use water heater allow for rapid heating of water at the point of use. This will reduce the cost for heating the entire water holding tank for the point of aeruginosa.
- Water quality guidelines: This risk assessment and risk characterization can serve as a starting place to set standards of P. aeruginosa concentrations in greywater. Per EPA guidelines, there are no set standards for P. aeruginosa. Scholarly research articles, standards and guidelines have been suggested. In Germany, the standard for P. aeruginosa is 100 CFU/ 100 mL, according to Winward et al..
Risk Perception
When scientific risk is communicated to the general public, there are many factors that contribute to how risk is perceived, according to Bruvold . Social and cultural influences will have a bias within the public. We want the actual risk to be communicated and not the perceived risk. The media also have an important role of relaying science to the public as well. As risk is accepted by the public, the mental model of reasoning is the state of thinking that assessors and informers want the general public to be; which is away from the mental model of intuition, which is based more on feeling.
Wastewater reuse is gaining popularity amongst the public due to water scarcity and more environmental efforts aimed at water preservation. However, negative notions of water reuse have limited the use of reclaimed water. Some ideals are “toilet to tap”, in the case of wastewater reuse. It is assumed that there is a greater risk from the reuse of water. Experts know the risk associated with high treated wastewater is low. In more recent years there has been a heightened awareness of drinking water and water quality general due to crises such as the Flint water crisis and the lead contamination in Washington D.C. water systems. This has lead to a lessened mistrust of water utilities and water regulatory agencies.
Specifically concerning P. aeruginosa, the general population is not knowledgeable about pseudomonas or its fate in the environment. Often times the general public’s view of risk may be different from that of risk assessors.
Risk Communication Strategy
To communicate the risk of P. aeruginosa high acceptance among the public is desired. This can be achieved by involving stakeholders at every stage of the risk assessment for a higher level of transparency and understanding among the public. Gurian divided risk perception into 3 areas: dread, familiarity, and number of people exposed to the risk, according to Gurian and Rose et al.. Dread can be defined as the intuitive response of the public to the risk. P. aeruginosa generally has a low dread factor due to its lack of popularity. As its name implies, familiarity is general knowledge about the risk and how well it is known by the public and relevance to everyday life. P. aeruginosa is well understood in scientific and medical communities. The last factor, number of people exposed to the risk is. For this risk assessment, the population of concern are military personnel. Because of the daily nature of showers, the entire FOB population is exposed to the risk.
Risk Communication
How to communicate the risk to military personnel and officials When communicating risk to the public, it should be expected that the perception of the scientific community and the public will vary. Ways to ensure that risk is properly communicated are to be accurate in sharing information. Being sure to state uncertainty, assumptions and limitations. The use of pictures and analogies can also be effective in communicating risk by simplifying complex ideas. Use of military lingo will allow for an increased understanding from military personnel: With every group of people there are terms and acronyms that are associated with the field. To reduce the risk by way of communicating steps can be taken to involve and engage the public on a risk. In this scenario, risk assessors and scientists will have to communicate risk to high ranking military personnel and soldiers who are most directly at risk as identified in the stakeholders portion of the report.
The presumed overall risk of military personnel is higher than that of civilians. Particularly for soldiers stationed at FOB’s. Soldiers are exposed to risk greater and more deadly risk than microbes such as warfare. However, this does not diminish the overall importance of reducing the risk of P. aeruginosa from dermal exposure.
References
-
(2016). Syria conflict: Obama to deploy 250 more special forces troops. Retrieved from http://www.bbc.com/news/world-middle-east-36126944
-
Dempsey, M. . (2015). Obama Looks at Adding Bases and Troops in Iraq, to Fight ISIS The New York Times. Retrieved from http://www.nytimes.com/2015/06/12/world/middleeast/iraq-isis-us-military-bases-martin-e-dempsey.html
-
(2015). Enhancing Stability and Security in Afghanistan. Arlington, Virginia: United States Department of Defense. Retrieved from http://www.defense.gov/Portals/1/Documents/pubs/1225_Report_Dec_2015_-_Final_20151210.pdf
-
Moore, T. . (2009). Report of the Afghanistan Marine Energy Assessment Team (MEAT). Retrieved from https://apps.dtic.mil/dtic/tr/fulltext/u2/a550136.pdf
-
(2011). Strategic Environmental Research and Development Program (SERDP): Sustainable Forward Operating Bases. Retrieved from https://iphc.amedd.army.mil/sites/EHE/DWSP/Shared%20Documents/SERDP_FOB_Report.pdf
-
(2014). PHIP No. 39-01-0514 Microbial Risk Assessment for Unrestricted Wastewater Reuse During Army Deployments. Retrieved from https://phc.amedd.army.mil/PHC%20Resource%20Library/PHIP-39-01-0514-MRA-WasteWaterReuse-May2014.pdf
-
(2011). Shower Water Reuse System to be force multiplier at FOBs. Retrieved from https://www.army.mil/article/65812/Shower_Water_Reuse_System_to_be_force_multiplier_at_FOBs/
-
Botzenhart, K. ., & Doring, G. . (1993). Ecology and epidemiology of Pseudomonas aeruginosa. Retrieved from https://link.springer.com/chapter/10.1007/978-1-4615-3036-7_1
-
Bédard, E. ., Boppe, I. ., Kouamé, S. ., Martin, P. ., Pinsonneault, L. ., Valiquette, L. ., … Prévost, M. . (2016). Combination of heat shock and enhanced thermal regime to control the growth of a persistent Legionella pneumophila strain. Pathogens, 5, 35. https://doi.org/10.3390/pathogens5020035
-
van Delden, C. . (2004). Virulence factors in Pseudomonas aeruginosa. Retrieved from https://link.springer.com/chapter/10.1007%2F978-1-4419-9084-6_1
-
Dembry, L. . (1998). Nosocomial bacterial infections. Retrieved from https://www.researchgate.net/publication/284074333_Nosocomial_Bacterial_Infections
-
System., N. N. (2004). National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004. Retrieved from https://www.cdc.gov/nhsn/pdfs/datastat/nnis_2004.pdf
-
Mena, K. . (2009). Risk assessment in Pseudomonas aeruginosa in water. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19484589
-
Rice, S. ., & Van den Akker, B. . (2012). A risk assessment of Pseudomonas aeruginosa in swimming pools: a review. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22717744
-
Zichichi, L. ., Asta, G. ., & Noto, G. . (2000). Pseudomonas aeruginosa folliculitis after shower/bath exposure. International Journal of Dermatology, 39, 270–273. https://doi.org/https://doi.org/10.1046/j.1365-4362.2000.00931.x
-
Hajjartabar, M. . (2004). Poor-quality water in swimming pools associated with a substantial risk of otitis externa due to Pseudomonas aeruginosa. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15318488
-
Washburn, J. ., & Jacobson, J. . (1976). Pseudomonas aeruginosa rash associated with a whirlpool. Retrieved from https://jamanetwork.com/journals/jama/article-abstract/345619
-
(2013). Antibiotic resistance threats in the United States. Retrieved from https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
-
Molina, D. . (1991). Unusual presentation of Pseudomonas aeruginosa infections: a review. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1816775
-
Khan, A. ., & Cerniglia, C. . (1994). Detection of Pseudomonas aeruginosa from clinical and environmental samples by amplification of the exotoxin A gene using PCR. Applied and environmental microbiology. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC201881/
-
(2005). Technical Manual. Retrieved from http://www.apd.army.mil
-
NATICK, I. . (2016). Force Provider. Retrieved from http://ilsc.natick.army.mil/forceprovider.htm
-
Sobsey, M. D., Stauber, C. ., Casanova, L. ., Brown, J. ., & Elliott, M. . (2008). Point of use household drinking water filtration: A practical, effective solution for providing sustained access to safe drinking water in the developing world. Environmental Science & Technology, 42, 12. https://doi.org/10.1021/es702746n
-
Winward, G. ., & Avery, L. . (2008). A study of the microbial quality of grey water and an evaluation of treatment technologies for reuse. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S092585740700211X
-
Hay, R. ., & Johns, N. . (2014). The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24166134
-
Roser, D. . (2015). Dose–response algorithms for water-borne Pseudomonas aeruginosa folliculitis. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25275553
-
Lautenschlager, K. ., & Boon, N. . (2010). Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Retrieved from https://www.sciencedirect.com/science/article/abs/pii/S0043135410005002
-
Pires, D. ., & Boas, D. . (2015). Phage Therapy: a Step Forward in the Treatment of Pseudomonas aeruginosa Infections. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25972556
-
(1998). What drives communities’ decisions and behaviours in the reuse of wastewater. Retrieved from https://www.academia.edu/31553564/What_drives_communities_decisions_and_behaviours_in_the_reuse_of_wastewater
-
Gurian, P. ., & Rose, J. . (2013). Theory and Practice of Quantitative Microbial Risk Assessment: An Introduction. Retrieved from http://qmrawiki.canr.msu.edu/images/6th_QMRA_Manual_2013.pdf