The Problem
Wastewater treatment plants along the Schuylkill River in southeastern Pennsylvania discharge small quantities of Cryptosporidium and Giardia oocysts into the river. The oocysts can then be transported down the river to recreational sites in Philadelphia. Here we investigate the risk associated with boating, fishing, wading, playing with water, and swimming in the Schuylkill River using Monte Carlo analysis. The mean risk of infection by Cryptoporidium and Giardia was determined to be 3.03E-07 and 2.07E-06, respectively. When standard treatment procedures are followed for waste treatment, recreational activities along the Schuylkill River pose little significant Cryptosporidiosis or Giardiasis threat to immunocompetent individuals. Nevertheless, sanitary education and access to sanitary facilities should be provided to the public.
Hazard Identification
Cryptosporidium spp
Cryptosporidium, also known as Crypto, is a microscopic parasite belonging to the genus Apicomplexan, according to Xiao. Some of the known important species include Cryptosporidium parvum and Cryptosporidium hominis. The microbes are ubiquitous in aquatic environments, and can be found in lakes, streams, and oceans, according to Weller. The oocyst is the infective form of the protozoa and is particularly hardy, resisting chlorine treatment, a common water treatment strategy, according to Centers for Disease Control. Cryptosporidium oocysts are transmitted by the fecal-oral pathway and common modes of transmission include ingesting recreational or treated water that has contacted the stool of an individual or animal that had Cryptosporidium, by eating uncooked food contaminated with Cryptosporidium, or by touching surfaces that have been contaminated with Cryptosporidium. Those who are commonly exposed to Cryptosporidium include children who attend daycare centers, childcare workers, international travelers, people who handle infected cattle, backpackers, hikers, and campers, people who swallow water from contaminated sources, and people who are exposed to human feces through sexual contact, according to Centers for Disease Control. Once ingested, an incubation period of between 4 to 8 days (Okhuysen et al) occurs after which an individual may develop cryptosporidiosis. Cryptosporidiosis is characterized by abundant watery diarrhea, sometimes accompanied by abdominal pain, nausea, vomiting, and fever. In immunocompetent individuals illness lasts 1-2 weeks then recovery usually occurs. However, in immunocompromised individuals, such as those with HIV, pregnant women, or in children under 5, diarrhea can be chronic and lead to severe complications, according to Weller. Studies estimate that Cryptosporidium has an attack rate that ranges from 52-86% (Okhuysen et al.), an estimated morbidity between 42% and 93% (Mac Kenzie et al.), and excretion rates of between 10^7 and 10^8 oocysts per bowel movement, according Yoder et al..
Giardia spp
Giardia, also known as Giardia intestinalis, Giardia lamblia or Giardia duodenalis, is a parasite that causes giardiasis, one of the most common parasitic diseases in the world, according to Weller. The parasite is found in every region in the U.S. and around the world, commonly affecting hiker and travelers inhabiting lakes, rivers, and streams, according to Weller. Giardia exists in the environment due to its outer shell, which can resist chlorine disinfection (Centers for Disease Control) and can remain viable for months in cold fresh water, according to Weller. The parasite is also transmitted by the fecal-oral route either by drinking contaminated water, by contacting infected surfaces, or due to recreational water activities, according to Weller. Once ingested, Giardia affects the upper part of the small intestine and after an incubation period of between 1 and 3 weeks (Zmirou-Navier et al and Weller) may manifest with stomach cramps, diarrhea, flatus, belching, fatigue, and nausea (Weller, 2012). Giardiasis often lasts 1 to 2 weeks or longer but can also persist for years, according to Weller. Giardia more severely affects young than old and newly exposed versus re-exposure. Moreover, illness can be more severe in patients with hypogammaglobulinemia and in AIDS patients, according to Weller. Studies have found a Giardia attack rate of 35% (Katz et al.), morbidity between 50 and 91% (Rose et al.), and estimated excretion rates on the order of 10^7 cysts per gram stool have been estimated, according to Yoder et al.
Exposure Assessment
Transport modeling for Cryptosporidium parvum and Giardia in Schuylkill River
This study assesses the impact of 72 waste treatment plants which discharge C. Parvum and Giardia into the Schuylkill River, given effluent concentrations. The first step is to determine the concentration of contaminants at the recreation site. A 1-D continuous mass input transport model was used to determine the concentration of Cryptosporidium and Giardia oocysts at the recreation area. Equation 1 indicates the change in concentration over the length of the river.
Equation 1
\begin{align*} \frac{C}{C_0} = \frac{1}{2}erfc[\frac{(x-vt)}{\sqrt{4E_xt}}]\times e^{-kt} \end{align*}
Since each treatment plant discharges waste at a different location on the river, the concentration was calculated for every segment of the river between treatment plants, x. Segment length x, velocity v, and time t were determined based on the dimensions and flow rate of the river. Dispersion coefficient Ex was estimated from the likeness of the Schuylkill River to rivers surveyed by the US EPA as indicated in Table 1.
| River | Dispersion Coefficient, Ex (m^2/s) |
|---|---|
| Chattahoochee River, GA | 32.5 |
| Susquehanna River, PA | 92.9 |
| Missouri River, NB-IA | 465 – 1487 |
| Antietam Creek, MD | 9.3 - 25.6 |
| Powell River, TN | 9.5 |
The decay constant k used is indicated in Table 2. When incorporating the decay rate constants for the protozoa into the transport model, several studies have found that the decay of Cryptosporidium is strongly influenced by water temperature (according to Jenkins et al and Robertson et al.), while changes in the decay rate with water temperature has not been reported for Giardia. In order to determine the decay rate constant for Cryptosporidium, the temperature of the Scuylkill River was estimated to be 16 C.
| Protozoa | k (days^-1) | Reference |
|---|---|---|
| C. Parvum | (10^-2.68)(10^0.58T) | Walker and Stedinger |
| Giardia | 2E-1 | Dorner et al. |
The discharge oocyst concentrations provided are listed in Table 3. The discharge concentrations for some waste water treatment plants were not reported. Absence of this data is designated by the entry of a dash (-) in Table 3. The WWTP facility number does not correspond to a specific facility, so matching discharge concentrations to facility was randomized in the Monte Carlo simulation as discussed in the Risk Characterization section.
Table 3: Concentrations of C. parvum and Giardia found in various WWTPs along the Schuylkill River
| WWTP Facility | 2007 Cryptosporidium (oocysts/L) | 2007 Giardia (cysts/L) |
| 1 | 0.093 | 0.28 |
| 2 | 0.35 | 0.78 |
| 3 | 0 | 0.273 |
| 4 | 0 | 8.27 |
| 5 | 0 | 0.4 |
| 6 | 0 | 6.17 |
| 7 | 0.12 | 0.35 |
| 8 | 0 | 0 |
| 9 | 1.68 | 8 |
| 10 | 0 | 0.4 |
| 11 | 0 | 0 |
| 12 | - | - |
| 13 | 0 | 72 |
| 14 | 0 | 1.48 |
| 15 | 0 | 2.5 |
| 16 | 0 | 87.2 |
| 17 | 0.27 | 1.64 |
| 18 | 0.19 | 0.48 |
| 19 | 0 | 15.13 |
| 20 | 0 | 0 |
| 21 | 0 | 3.64 |
| 22 | 0.083 | 0.42 |
| 23 | 0 | 3.01 |
| 24 | 0 | 2.61 |
| 25 | 0 | 1.91 |
| 26 | 0 | 1.86 |
| 27 | 0 | 0.9 |
| 28 | 0.089 | 7.77 |
| 29 | 0 | 0 |
| 30 | 0 | 5.83 |
| 31 | - | - |
| 32 | 0 | 0.087 |
| 33 | 0.18 | 8.55 |
| 34 | 0.76 | 1.62 |
| 35 | 0.1 | 1.1 |
| 36 | 0.083 | 0.75 |
| 37 | 1.73 | 0.82 |
| 38 | 0 | 4.34 |
| 39 | 0 | 15.58 |
| 40 | 0 | 30.9 |
| 41 | 0.083 | 0.083 |
| 42 | 0.61 | 8.87 |
| 43 | 0 | 0.087 |
| 44 | 0 | 0 |
| 45 | - | - |
| 46 | 0 | 16.5 |
| 47 | 0.64 | 0.82 |
| 48 | 0 | 0.7 |
| 49 | 0.44 | 2.26 |
| 50 | 2.39 | 0 |
| 51 | 0 | 4.33 |
| 52 | 0 | 0 |
| 53 | 0 | 7.83 |
| 54 | 0.27 | 0.273 |
| 55 | 0 | 0.2 |
| 56 | 1.64 | 0.182 |
| 57 | 0.17 | 28.3 |
| 58 | 0 | 0.444 |
| 59 | 0 | 0.091 |
| 60 | 0 | 0 |
| 61 | 0 | 0 |
| 62 | 0 | 0 |
| 63 | 0 | 0.087 |
| 64 | 0 | 3.92 |
| 65 | - | - |
| 66 | 0 | 0 |
| 67 | 0 | 0.667 |
| 68 | 0.18 | 0 |
| 69 | 0 | 16.73 |
| 70 | 0 | 0 |
| 71 | 0.087 | 0.174 |
| 72 | 0 | 0.182 |
Dose Response
Exponential Dose Response model
The dose of a pathogen that is received due to a particular activity can be calculated as the product of the concentration of pathogen at the recreation site (cyst/L), the activity ingestion rate (mL/hr), and the duration of exposure (hr), as indicated in Equation 2. A dose response model can then be used to calculate the risk of infection due to the dosage received. An exponential model (Equation 3) has been used to describe the dose response of individuals for both Cryptosporidium (Rendtorff 1954) and Giardia (Messner et al 2001); and was utilized in this investigation to calculate the risk of infection. The probability an illness will result from an infection can then be modeled assuming a constant risk of illness for a particular pathogen (Equation 4). Assuming that the risk of mortality is known and constant we can also forecast the probability a mortal infection will occur due to this exposure event (Equation 5).
Equation 2
\begin{align*} Dose = Concentration * Ingestion \ Rate * Exposure Duration \end{align*}
Equation 3
\begin{align*} P(Infection) = 1 - e^{-k * Dose} \end{align*}
Equation 4
\begin{align*} P(Illness | Infection) = P(Infection) * Risk \ of \ illness \end{align*}
Equation 5
\begin{align*} P(Mortality | Illness) = P(Ilness | Infection) * Risk \ of \ Mortality \end{align*}
The dose response parameters, risk of illness, risk of mortality parameters used for this model are presented in Table 4 below.
Table 4. Parameters for dose, infection, illness, and mortality calculations
| Cryptosporidium | Giardia | |
| Dose Response Parameter (k) | 5.72E-02 (a) | 1.99E-02 (b) |
| P(Illness,Infected) | 0.675 | 0.705 |
| P(Mortality,Illness) | 1E-04 | 1E-04 |
a Messner et al., 2001
b Rendtorff, 1954
Risk Characterization
A Monte Carlo Model was used to carry out the Risk Characterization. The model was built and designed using Crystal Ball and Microsoft Excel. The model was run for 100,000 cycles, generating 3 risk outputs (Risk of Infection, Risk of Illness, and Risk of mortality) for both pathogens. The concentration of pathogens in the wastewater effluent and the rate of ingestion of river water by activity were selected from model distributions as part of the Monte Carlo simulation. The duration of exposure events were modeled as fixed and known as indicated in Table 4. It was assumed that the concentration of Cryptosporidium and Giardia in wastewater effluent was identical for each of the 72 wastewater treatment plants. This assumption was made in order to greatly reduce the computational demands of the model when it was run. The Cryptosporidium and Giardia concentrations in Table 3 were used as model distributions for the effluent concentrations. Each cycle of the Monte Carlo Model selected a value from the two distributions and that concentration was applied to all effluent concentrations in the transport portion of the model. Figures 1 and 2 below show the distribution of oocysts and cysts in wastewater effluent entering the Schuylkill River.
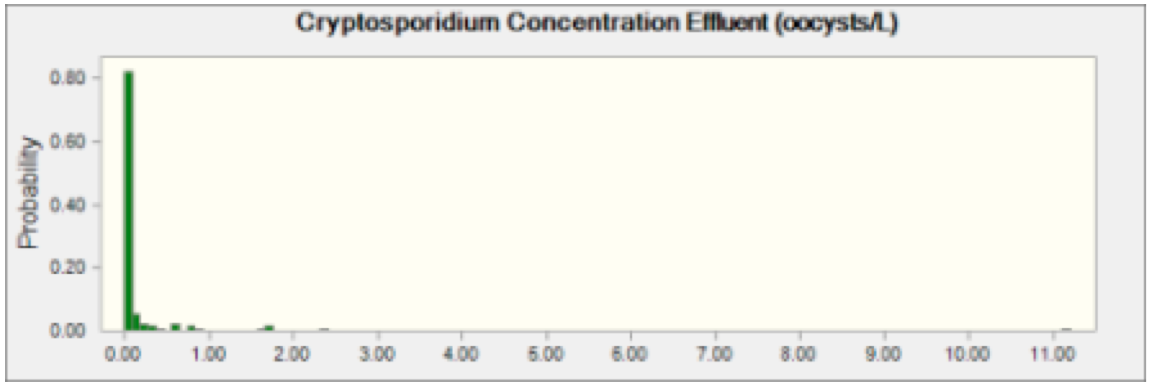
Figure 1 Cryptosporidium Effluent Concentration This indicates probability of oocysts concentration of Cryptosporidium in effluent coming from any plant discharging into the Schuylkill River.
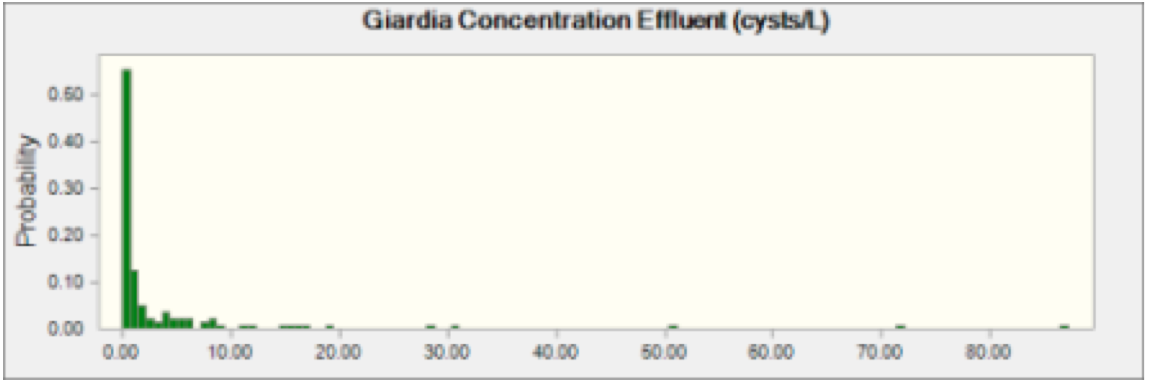
Figure 2 Giardia Effluent Concentration This indicates probability of oocysts concentration of Giardia in effluent coming from any plant discharging into the Schuylkill River.
The effects of dilution, diffusion, dispersion, and decay were then taken into account in the transport portion of our model, as discussed in the Exposure Assessment section. This allowed us to forecast the concentration of pathogens in the river water at the recreational site (Figure 3 and 4).
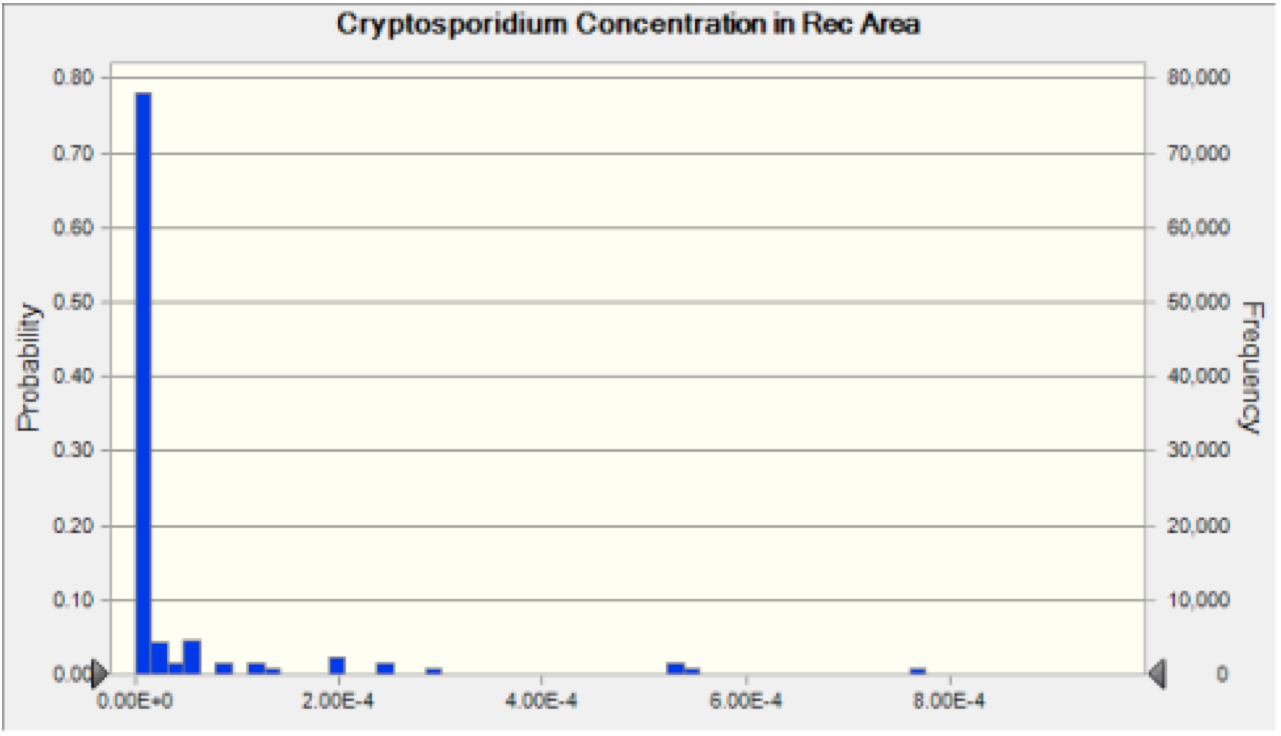
Figure 3 Cryptosporidium Recreation Area Concentration The probability distribution of concentration of oocysts that results from the discharge of Cryptosporidium into the river at each plant. This result is then used to calculate the risk associated with ingestion of water at the recreation area.
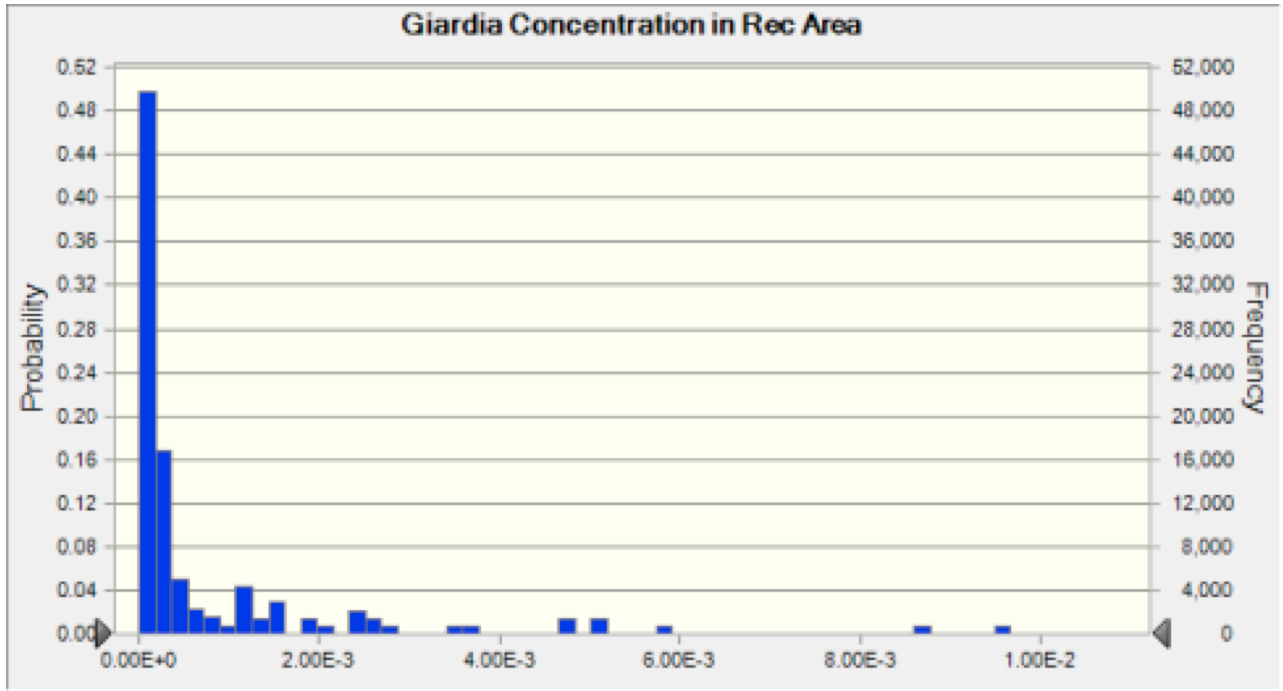
Figure 4 Giardia Recreation Area Concentration The probability distribution of concentration of oocysts that results from the discharge of Giardia into the river at each plant. This result is then used to calculate the risk associated with ingestion of water at the recreation area.
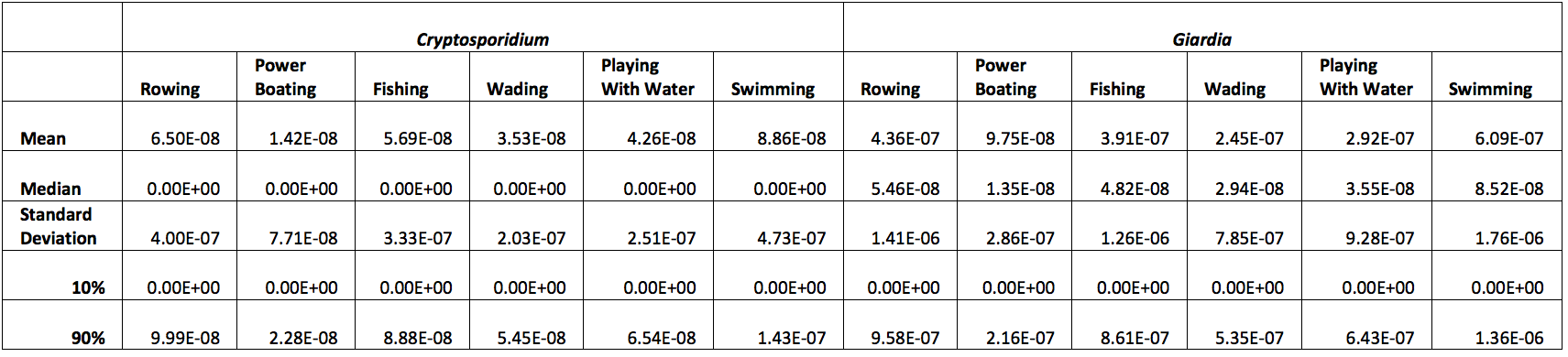
Each cycle of the model also selected an ingestion rate for each activity from the activities respective distribution. With this ingestion rate and the modeled concentration of pathogen, Dose, Risk of Infection, Risk of Illness, and Risk of Mortality can then be calculated deterministically. The resulting statistics for the Risk of Infection are presented in Table 5 below. It should be emphasized that these risk values are per activity/event and are independent of other risks; as such, engaging in multiple activities for increasing the duration of exposure will result in an associated Risk of Infection.
Table 5: Calculated Risk of Infection
We can see that the Risk of Infection for each of these activities is projected to be very small when compared to a commonly used cutoff of E-04.The low Risk of Infection values that were generated by the model arise from the low concentrations of cysts and oocycsts in wastewater effluent that is entering the river, and diffusion, dispersion, and settling serve to further decrease pathogen concentrations by the time the river water reached the recreational area. Additionally, we can observe that the Risk of Infection by Giardia is always higher than the risk of infection by Cryptosporidium. This is most likely due to the higher concentration of Giardia cysts in wastewater plant effluent. This conclusion is supported by the fact that Cryptosporidium has a larger dose response parameter and a smaller decay rate parameter in the transport portion of the model.
Limitations of Model
There are several limitations to the current conceptual model. The most significant is that wastewater effluent is not the only source of parasites in surface waters; the addition of other vectors for contamination such as urban and rural runoff would likely result in the model raising Cryptosporidium and Giardia concentrations. In particular, runoff from agricultural sites with livestock may serve to greatly increase these concentrations. The Schuylkill River is also subjected to releases of untreated wastewater during periods of heavy precipitation. This is the result of the combined sewer system that is in use and limited waste water treatment plant capacity. These releases could greatly increase the concentrations of pathogens present in the river. These releases are not taken into account in the current model.
There are also limitations within the application of the model. River temperature, a variable that helps determine dispersion/diffusion and decay rate was modeled as a constant. River temperature is variable over the course of a year and that may have an effect that was not measured in this analysis. Additionally, each of the 73 plants could be modeled with independent effluent concentrations. The decision to reduce 73 plant effluent concentrations to 1 identical concentration was done to avoid crashes within Crystal Ball and Excel. Utilizing a command line based programming language such as Python for the Monte Carlo simulation would likely reduce these issues and allow more variation to be modeled without technical concerns. Lastly, exposure durations were modeled as point estimates. It is likely that modeling these durations as distributions would increase the variation observed in the results.
Risk Management
From our model we conclude that the recreational activities along the Schuylkill River pose little significant Cryptosporidiosis or Giardiasis threat to immunocompetent individuals as long as the effluent treatment protocols are observed. However, vulnerable populations may have a higher likelihood of developing Cryptosporidiosis or Giardiasis.
Currently, there is some debate about the level of exposure risk for either Cryptosporidium or Giardia species and there has not yet been an established agreeable threshold above which one could predict illness. It has been suggested that in treated drinking water a concentration of 3-5 cysts per 100L for Giardia (Wallis et al., 1996) and 10-30 oocysts per 100L for Cryptosporidium (Haas and Rose, 1995) could potentially serve as a threshold. Meanwhile, other studies suggest that rather than estimating risk in an outbreak it may be easier to identify the species and genotype and whether it is infectious prior to taking any action (Robertson et al., 2009). Further study is needed to elucidate this issue.
Recreational water bodies, however, present a different set of challenges when assessing risk. A recreational water site can include “swimming, walking trails, canoeing, fishing, water skiing, marroning, camping and caravan” (Sasdekumar et al., 2012). As these sites may serve as pre-treatment areas, they can often be contaminated with Cryptosporidium via “farm animal sewage, human sewage discharge and contamination from wild animals.” (Bryan et al., 2009;Xiao, 2010) For these sites then, studies have suggested that water quality monitoring be used prior to taking action (Centers for Disease Control and Prevention, 2008). For instance, the Environmental Protection Agency has suggested that the “monthly geometric mean water-quality indicator concentration be ≤33 CFU/100mL for enterococci or ≤126 CFU/100 mL for E. Coli.” (Centers for Disease Control and Prevention, 2008) Moreover, human behavior is also linked to risk of exposure to illness as it has been shown that there is an association between the prevalence of Cryptosporidium species in recreational waters and population density (Sasdekumar et al., 2012). Given that both Giardia and Cryptosporidium species are transmitted via the fecal-oral route, it would make sense to predict an elevated risk to persons who are in close proximity to individuals who have diarrhea or who practice poor hygiene.
While an acceptable level of risk is debatable, available risk-reducing plans are imperative, particularly for vulnerable populations. For instance, if monthly water quality thresholds have been exceeded, state and local authorities could either place placards at the site to warn visitors nearby or restrict access to the recreational area entirely (Centers for Disease Control and Prevention, 2008). In addition, to reduce exposure to potential contamination from runoff upstream, authorities can restrict access to the recreational area after a rainfall event or prohibit recreational activity near storm drains (Centers for Disease Control and Prevention, 2008). In order to address human sources of exposure, it is recommended that proper hygienic facilities, such as diaper-changing stations, handwashing stations, showers and toilets be present near recreation areas (Centers for Disease Control and Prevention, 2008). Moreover, patrons should be educated regarding the need to shower prior to entering the water source, to avoid swallowing water and to keep away from bathing altogether if they have diarrhea (Centers for Disease Control and Prevention, 2008).
Regardless of preventive measures, if an outbreak of illness in a recreational area within the confines of the Schuylkill River were to occur, it is crucial that we inform immunocompromised individuals as soon as possible so that they can avoid the site. We recommend alerting both social media to get this message out quickly and having physicians contact their patients directly to cover those who may not have social media access. In order to allay concerns among immunocompetent individuals, we would also suggest making a public service announcement via the media that clearly emphasized that immunocompetent individuals have less of a risk of developing serious illness. Lastly, the water effluent treatment companies are encouraged to maintain surveillance and to develop early warning systems to ensure efficiency of communication in case of any future occurrences that may overwhelm current capabilities. This communication process needs to be monitored by the Philadelphia Department of Public Health to determine how the public responds and if more public attention is needed.
The recreational activities along the Schuylkill River pose little significant cryptosporidiosis or giardiasis threat to immunocompetent individuals. The river remains safe as long as the effluent treatment protocols are observed. However, vulnerable populations may have a higher likelihood of developing cryptosporidiosis.
1. Bryan, B.A., Kandulu, J., Deere, D.A., White, M., Frizenschaf, J., Crossman, N.D., 2009. Adaptive management for mitigating Cryptosporidium risk in source water: a case study in an agricultural catchment in South Australia. Journal of Environmental Management. 90 (10), 3122–3134.
2. Centers for Disease Control (2012) Cryptosporidiosis. Retrieved from . http://www.cdc.gov/parasites/crypto.
3. Centers for Disease Control (2012). Parasites - Giardia. Retrieved from http://www.cdc.gov/parasites/giardia/.
4. Centers for Disease Control and Prevention. Surveillance for Waterborne Disease and Outbreaks Associated with Recreational Water Use and Other Aquatic Facility-Associated Health Events — United States, 2005–2006 and Surveillance for Waterborne Disease and Outbreaks Associated with Drinking Water and Water not Intended for Drinking — United States, 2005–2006. Surveillance Summaries, [September 12]. MMWR 2008; 57(No. SS-9).
5. Dorner, S.M., Anderson, W.B., Slawson, R.M., Kouwen, N., Huck, P.M., 2006. Hydrologic modeling of pathogen fate and transport. Environmental Science and Technology 40: 4746 – 4753.
6. Dufour, A.P., Evans, O.E., Behymer, T.D., Cantú, R., 2006. Water ingestion during swimming activities in a pool: A pilot study. Journal of Water Health, 04.4, 425-430.
7. Geosyntec, 2008. Dry and Wet Weather Risk Assessment of Human Health Impacts of Disinfection vs. No Disinfection of the Chicago Area Waterways System (CWS). Chicago.
8. Haas, C.N., Rose, J. B., 1995. Developing an anaction level for Cryptosporidium. American Water Works Association, 87(9), 81-84.
9. Haas, C.N., Rose, J. B., & Gerba, C. P. (1999). Quantitative microbial risk assessment, John Wiley & Sons, Inc. New York, NY.
10. Jenkins, M.B., Anguish, L., Bowman, D., Walker, M., Ghiorse, W., 1997. Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Applied Environmental Microbiology 63: 3844– 50.
11. Katz, D.E., Heisey-Grove, D., Beach, M., Dicker, R.C., Matyas, B.T. (2006). Prolonged outbreak of giardiasis with two modes of transmission. Epidemiology and Infection, 134(5), 935-941.
12. MacKenzie, W.R., Hoxie, N. J., Proctor, M. E., Gradus, M. S., Blair, K. A., Peterson, D. E., Kazmierczak, J.J., Addiss, D.G., Fox, K.R., Rose, J.B., Davis, J.P. (1994). A Massive Outbreak in Milwaukee of Cryptosporidium Infection Transmitted through the Public Water Supply. NEJM, 331, 161-167.
13. Messner, M.J., Chappell, C.L., Okhuysen, P.C. (2001). Risk assessment for Cryptosporidium: A hierarchical Bayesian analysis of human dose response data, 35(16), 3934-3940.
14. Okhuysen, P.C., Chappell, C.L., Crabb, J.H., Sterling, C.R., DuPont, H.L. (1999). Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. The Journal of Infectious Diseases, 180(4), 1275-1281.
15. Philadelphia Department of Public Health (PDPH). (2011). Division of Disease Control 2011 Annual Report. http://www.phila.gov/health/pdfs/PDPH_DiseaseControl_AnnualReport_2011.pdf.
16. Regli, S., Rose, J. B., Haas, C. N., & Gerba, C. P. (1991). Modeling the risk from giardia and viruses in drinking water. Journal of American Water Works Association, 83, 76-84.
17. Rendtorff, R.C. (1954). The experimental transmission of human intestinal protozoan parasites. II. Giardia lamblia cysts given in capsules. American Journal of Epidemiology, 59(2), pp.209-220.
18. Robertson, L.J., Campbell, A.T., Smith, H.V., 1992. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Applied Environmental Microbiology 58: 3494 – 500.
19. Robertson, L., Gjerde, B., Hansen, E.F., Stachurska-Hagen, T. (2009). A water contamination incident in Oslo, Norway during October 2007; a basis for discussion of boil-water notices and the potential for post-treatment contamination of drinking water supplies. Journal of Water and Health, 07(1), 55-66.
20. Rose, J.B., Haas, C.N., Regli, S. (1991). Risk assessment and control of waterborne giardiasis. American Journal of Public Health, 81(6), 709-713.
21. Sasdekumar Loganthan a, Rongchang Yang a, Andrew Bath b, Cameron Gordon b, Una Ryan. (2012). Prevalence of Cryptosporidium species in recreational versus non-recreational water sources. J. Experimental Parasitology. 131, 399-403. http://dx.doi.org/10.1016/j.exppara.2012.04.015
22. Sunger, Neha. (2013). Quantitative Health Risk Assessment of Recreational Water Users in Philadelphia. (Doctoral Dissertation). Retrieved from http://hdl.handle.net/1860/4104.
23. United States Environmental Protection Agency, 2000. Health Effects Criteria for Fresh Recreational Waters. Cincinnati, Ohio, U.S. Environmental Protection Agency. T. A. M. Department. Cincinnati, USEPA
24. United States Environmental Protection Agency, 2013. Dispersions and Exchanges. Watershed and Water Quality Technical Support Center, Washington, D.C. Retrieved December 19, 2013. http://www.epa.gov/athens/wwqtsc/courses/wasp7/transport/Dispersion.ppt
25. Walker, F.R., Stedinger, J.R., 1999. Fate and transport model of Cryptosporidium. Journal of Environmental Engineering 125: 325 – 333.
26. Wallis, P.M., Erlandsen, S. L., Isaac-Renton, J. L., Olson, M. E., Robertson W J. and van Keulen, H. (1996). Prevalence of Giardia cysts and Cryptosporidium oocysts and characterization of Giardia spp. isolated from drinking water in Canada. Appl. Environ. Microbiol. 62(8), 2789-2797.
27. Weller P.F. (2012). Chapter 215. Protozoal Intestinal Infections and Trichomoniasis. In D.L. Longo, A.S. Fauci, D.L. Kasper, S.L. Hauser, J.L. Jameson, J. Loscalzo (Eds), Harrison's Principles of Internal Medicine, 18e. Retrieved December 18, 2013 from http://www.accessmedicine.com/content.aspx?aID=9125604.
28. Xiao, G., Qiu, Z., Qi, J., Chen, J., Liu, F., Liu, W., Luo, J., Shu, W., 2013. Occurrence and potential health risk of Cryptosporidium and Giardia in the Three Gorges Reservoir, China. Water Research 47: 2431-2445http://www.ncbi.nlm.nih.gov/pubmed?term=Xiao L%5BAuthor%5D&cauthor=true&cauthor_uid=14726456
29. Xiao, L., 2010. Molecular epidemiology of cryptosporidiosis: an update. Experimental Parasitology. 124, 80–89.
30. Xiao L, Fayer R, Ryan U, Upton SJ (2004) Cryptosporidium taxonomy: recent advances and implications for public health. Clin Microbiol Rev.
31. Yoder, J.S., Wallace, R.M., Collier, S.A., Beach, M.J., Hlavsa, M.C. (2012). Cryptosporidiosis Surveillance – United States, 2009-2010. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6105a1.htm.
32. Zmirou-Navier D, Gofti-Laroche L and Hartemann P (2006) Waterborne microbial risk assessment: a population-based dose-response function for Giardia spp.(E.MI.R.A study). BMC Public Health, 6 (1), 122.
References
-
Xiao, L. ., Fayer, R. ., & Ryan, U. . (2004). Cryptosporidium taxonomy: recent advances and implications for public health. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14726456
-
Weller, P. . (2012). Protozoal Intestinal Infections and Trichomoniasis. Retrieved from https://accessmedicine.mhmedical.com/Content.aspx?bookid=1130§ionid=79740785
-
(2012). Cryptosporidiosis. Retrieved from https://www.cdc.gov/parasites/crypto/index.html
-
Okhuysen, P. ., Chappell, C. ., Crabb, J. ., & Sterling, C. . (1999). Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10479158
-
MacKenzie, W. ., Hoxie, N. ., Proctor, M. ., Blair, K. ., Peterson, D. ., Kazmierczak, J. ., & Addiss, D. . (1994). A Massive Outbreak in Milwaukee of Cryptosporidium Infection Transmitted through the Public Water Supply. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7818640
-
Yoder, J. ., Wallace, R. ., Collier, S. ., Beach, M. ., & Hlavsa, M. . (2012). Cryptosporidiosis Surveillance – United States, 2009-2010. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6105a1.htm
-
Prevention, C. for D. C. (2015). Parasites- Giardia General Information. Retrieved from http://www.cdc.gov/parasites/giardia/general-info.html
-
Zmirou-Navier, D. ., Gofti-Laroche, L. ., & Hartemann, P. . (2006). Waterborne microbial risk assessment: a population-based dose-response function for Giardia spp.(E. MI. RA study). BMC Public Health, 6. https://doi.org/10.1186/1471-2458-6-122
-
Katz, D. ., Heisey-Grove, D. ., Beach, M. ., Dicker, R. ., & Matyas, B. . (2006). Prolonged outbreak of giardiasis with two modes of transmission. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16569269
-
Rose, J. ., Haas, C. ., & Regli, S. . (1991). Risk assessment and control of waterborne giardiasis. American Journal of Public Health, 81(6), 709–713. https://doi.org/10.2105/ajph.81.6.709
-
Jenkins, M. ., Anguish, L. ., Bowman, D. ., Walker, M. ., & Ghiorse, W. . (1997). Assessment of a dye permeability assay for determination of inactivation rates of Cryptosporidium parvum oocysts. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9327547
-
Robertson, L. J., Campbell, A. T., & Smith, H. . , V. (1992). Survival of Cryptosporidium parvum oocysts under various environmental pressures. Applied and Environmental Microbiology, 58.
-
Walker, F. ., & Stedinger, J. . (1999). Fate and transport model of Cryptosporidium. Journal of Environmental Quality, 33.
-
Dorner, S. ., Anderson, W. ., Slawson, R. ., Kouwen, N. ., & Huck, P. . (2006). Hydrologic modeling of pathogen fate and transport. Retrieved from https://pubs.acs.org/doi/10.1021/es060426z