General overview
Acanthamoeba spp. are free-living amoeba (FLA) that have been commonly found in freshwater, tap water, and recreational water. Acanthamoeba spp. are capable of causing a variety of infections, including Acanthamoeba keratitis, an eye infection that has been associated with contect lens usage and cornea damage, and Granulomatous Amebic Encephalitis (GAE), a serious central nervous system infection that primarily affects immunocompromised individuals (Marciano-Cabral & Cabral, 2003; Visvesvara, Moura, & Schuster, 2007)[2] . Considering these infections, some exposure routes of concern for Acanthamoeba spp. are the corneal and intranasal exposure routes.
Dean et al. (2020)[1] fit dose response models to data from previously conducted animal studies for Acanthamoeba spp. and the corneal and instranasal exposure routes. These models are an important step towards characterizing the risk associated with FLA like Acanthamoeba for drinking water-relevant exposure scenarios. More detailed descriptions of the datasets, fitting methods, model evaluation, and results can be found in the published article: http://dx.doi.org/10.1111/risa.13603
Recommended Model
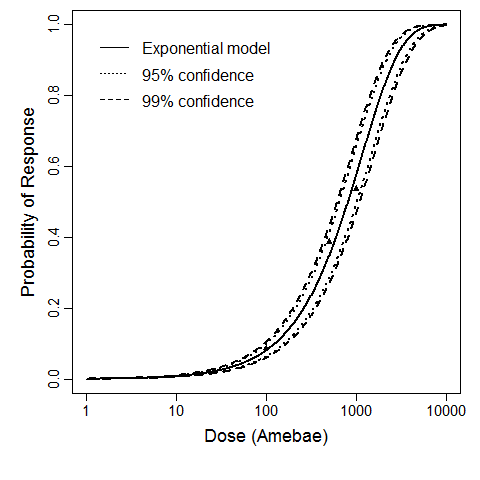
For the intranasal exposure route, the exact beta-Poisson model fit to the pooled data from Experiments 3 and 4 is the recommended model. The successful pooling of the model gives additional confidence for applying the dose response relationship to additional Acanthamoeba strains and species.
[2] Marciano-Cabral, F., & Cabral, G. (2003). Acanthamoeba spp. as agents of disease in humans. Clinical Microbiology Reviews. https://doi.org/10.1128/CMR.16.2.273-307.2003
| ID | Exposure Route | # of Doses | Agent Strain | Dose Units | Host type | Μodel | LD50/ID50 | Optimized parameters | Response type | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Acanth_Intranasal1 | intranasal | 6.00 | A. culbertsoni (A1) | no of trophozoites | mice | beta-Poisson | a = 0.161 N50 = 14,690 or 14,538 | death | ||
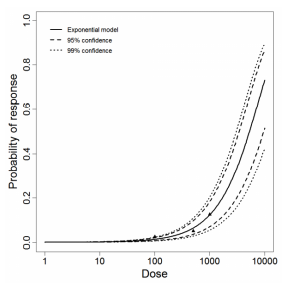
| Acanth_Intranasal2 | intranasal | 3.00 | A. castellanii HN-3 | no of trophozoites | mice | exponential | 5.28E+03 | k = 1.31E-04 | death |
Culbertson, C. ., Ensminger, P. ., & Overton, W. . (1966). Hartmannella (acanthamoeba). Experimental chronic, granulomatous brain infections produced by new isolates of low virulence. American Journal of Clinical Pathology, 46, 305–314. |
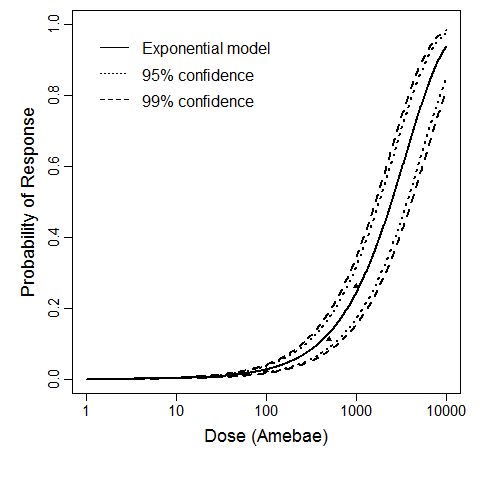
| Acanth_Intranasal3 | intranasal | 3.00 | A. castellanii HN-3 | no of trophozoites | mice | exponential | 749 | k = 9.26E-04 | brain invasion |
Culbertson, C. G., Ensminger, P. W., & Overton, W. M. (1966). Hartmannella (acanthamoeba). Experimental chronic, granulomatous brain infections produced by new isolates of low virulence. American Journal of Clinical Pathology, 46, 305-314. |
| Acanth_Intranasal4 | intranasal | 3.00 | A. castellanii HN-3 | no of trophozoites | mice | exponential | 2.67E+03 | k = 2.60E-04 | acute meningoencephalitis |
Culbertson, C. ., Ensminger, P. ., & Overton, W. . (1966). Hartmannella (acanthamoeba). Experimental chronic, granulomatous brain infections produced by new isolates of low virulence. American Journal of Clinical Pathology, 46, 305–314. |
| Acanth_Intranasal_Pooled | intranasal | 9.00 | A. castellanii HN-3 and A culbertsoni A1 | no of trophozoites | mice | beta-Poisson | a = 0.245 N50 = 19357 | death |
Červa (1967a,b) studied white mice of the Czechoslovak H-strain weighing 13-15 grams inoculated intranasally by placing 0.02 mL of the A1 strain of Acanthamoeba over the nares of the ethyl-ether anesthetized mice (Cerva, 1967b).
The beta-Poisson model provided the best fit to the data.
Cerva, L. (1967b). Intranasal, Intrapulmonary, and Intracardial Inoculation of Experimental Animals with Hartmanella castellanii. Folia Parasitologica (Praha), 14, 207–215.
Culbertson et al. (1966) studied the pathogenicity of the HN-3 strain of A. castellanii (Culbertson et al., 1966; Marciano-Cabral & Cabral, 2003) on ether-anesthetized-specific-pathogen-free (SPF) mice. Cultures of amebae were grown in trypticase soy broth and diluted so that 0.03 mL of a concentrated suspension could be instilled intranasally into the mice by placing fluid over the nares (Culbertson et al., 1966; Culbertson, Ensminger, & Overton, 1965a; Culbertson, Ensminger, & Overton, 1965b).
The exponential model provided the best fit to the data.
Culbertson, C. G., Holmes, D. H., & Overton, W. M. (1965b). Hartmanella castellani (Acanthamoeba sp.). The American Journal of Clinical Pathology, 43(4), 361–364.
Culbertson, C. G., Ensminger, P. W., & Overton, W. M. (1966). Hartmannella (Acanthamoeba): Experimental Chronic, Granulomatous Brain Infections Produced by New Isolates of Low Virulence. The American Journal of Clinical Pathology, 46(3), 305–314.
Culbertson et al. (1966) studied the pathogenicity of the HN-3 strain of A. castellanii (Culbertson et al., 1966; Marciano-Cabral & Cabral, 2003) on ether-anesthetized-specific-pathogen-free (SPF) mice. Cultures of amebae were grown in trypticase soy broth and diluted so that 0.03 mL of a concentrated suspension could be instilled intranasally into the mice by placing fluid over the nares (Culbertson et al., 1966; Culbertson, Ensminger, & Overton, 1965a; Culbertson, Ensminger, & Overton, 1965b).
The exponential model provided the best fit to the data.
Culbertson, C. G., Holmes, D. H., & Overton, W. M. (1965b). Hartmanella castellani (Acanthamoeba sp.). The American Journal of Clinical Pathology, 43(4), 361–364.
Culbertson, C. G., Ensminger, P. W., & Overton, W. M. (1966). Hartmannella (Acanthamoeba): Experimental Chronic, Granulomatous Brain Infections Produced by New Isolates of Low Virulence. The American Journal of Clinical Pathology, 46(3), 305–314.
Culbertson et al. (1966) studied the pathogenicity of the HN-3 strain of A. castellanii (Culbertson et al., 1966; Marciano-Cabral & Cabral, 2003) on ether-anesthetized-specific-pathogen-free (SPF) mice. Cultures of amebae were grown in trypticase soy broth and diluted so that 0.03 mL of a concentrated suspension could be instilled intranasally into the mice by placing fluid over the nares (Culbertson et al., 1966; Culbertson, Ensminger, & Overton, 1965a; Culbertson, Ensminger, & Overton, 1965b).
The exponential model provided the best fit to the data.
Culbertson, C. G., Ensminger, P. W., & Overton, W. M. (1966). Hartmannella (Acanthamoeba): Experimental Chronic, Granulomatous Brain Infections Produced by New Isolates of Low Virulence. The American Journal of Clinical Pathology, 46(3), 305–314.
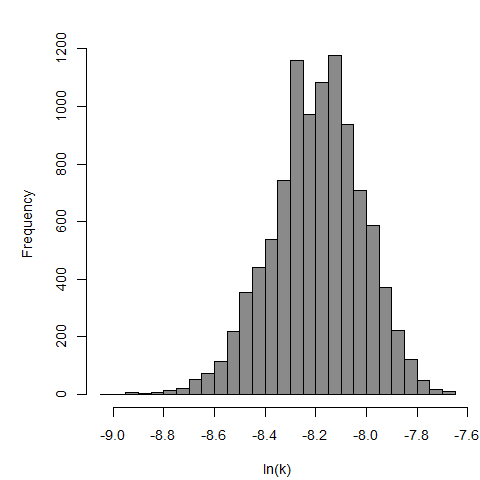
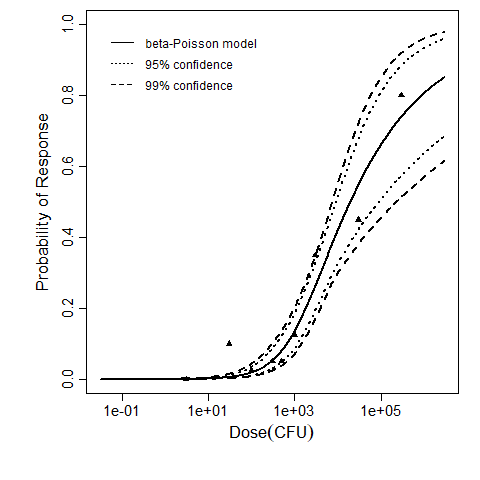
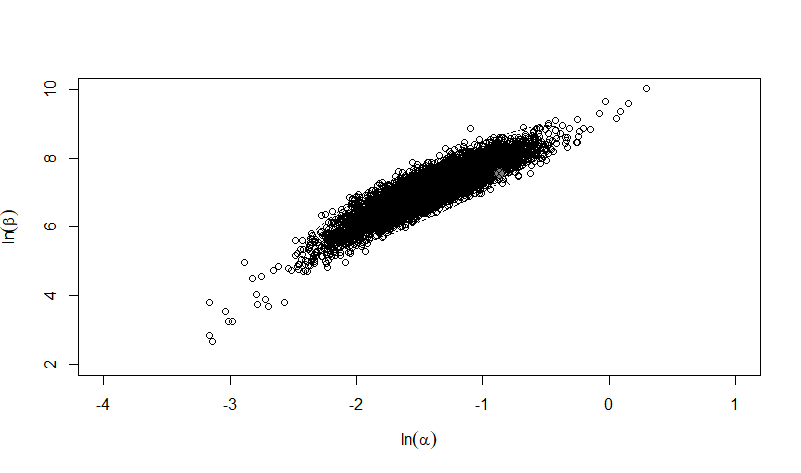
The same exposure route and endpoint was evaluated for Experiments 3 and 4 (Cerva, 1967b; Culbertson et al. 1966)[6] [5]. A pooling analysis was attempted and successful. The beta-Poisson model provided a good fit to the pooled data and is shown below in Figure 1. Note: both the exact and approximate beta-Poisson models were fit to the data. The figures shown below and the csv file of bootstrapped parameter replicates are for the best fitting parameters of the exact beta-Poisson model. The successful pooling of multiple datasets generally increases the confidence in the estimated model parameters.
[6] Cerva, L. (1967b). Intranasal, Intrapulmonary, and Intracardial Inoculation of Experimental Animals with Hartmanella castellanii. Folia Parasitologica (Praha), 14, 207–215.
[5] Culbertson, C. G., Ensminger, P. W., & Overton, W. M. (1966). Hartmannella (Acanthamoeba): Experimental Chronic, Granulomatous Brain Infections Produced by New Isolates of Low Virulence. The American Journal of Clinical Pathology, 46(3), 305–314.